Instrumental Swallow Evaluation
 Instrumental Diagnostic Procedure
Instrumental Diagnostic Procedure
An instrumental examination may be performed after or instead of a clinical exam. A clinical exam should trigger an instrumental procedure if (1) reported symptoms are inconsistent with the findings on the clinical exam, (2) further differential diagnosis is needed to refine the clinical hypothesis or determine appropriate treatment, (3) further information is needed to improve swallow safety, or (4) patient has significant pulmonary compromise and there is a need to rule out any aspects of the swallow deficit that can exacerbate the compromised respiratory function. A patient should NOT receive an instrumental exam if they are not medically stable enough to tolerate the procedure including transporting them to the instrumentation (if needed) or if the instrumental exam will not enhance the knowledge of the swallow deficit or guide in the treatment direction.
Clinical Note
When choosing an instrumental exam consider the following:
- When choosing VFES, consider the risk:benefit ratio of radiation exposure.
a. For VFES, if the acquisition pulse or frame rate is inadequate to answer the clinical questions, then the radiation exposure may not be worthwhile. - How important is it to your evaluation to include the entire system and to observe bolus flow?
- What is the importance of the laryngeal exam? Is laryngeal visualization important?
- How important is it to include the oral cavity and oral bolus manipulation?
a. Does oral opening alter the ability to evaluate the oral cavity during the clinical exam? - Would you like to visualize the cervical esophagus?
a. Are you hoping to differentiate between passive and active UES forces? - Any recent history of nasal surgery? History of fainting?
- Do you need to evaluate velopharyngeal closure?
- Can the patient be transported to the fluorography suite?
- What is the availability of the fluorography suite? Could a FEES be scheduled sooner?
- Has a recent VFES been performed?
- Suspicion of aspiration on secretion.
Activity
Activity 3.2
TASK: Build a mind map that links the concerns listed in the clinical note to the various instrumental exam. In other words, what instrumental exam would you choose if the answer the question was no? If the answer was yes? Why? See Appendix 2 for an example.
Instrumental approaches aid in objectifying and/or visualizing biomechanical and temporal events, as well as pressure gradients associated with bolus flow during swallowing. This information allows the dysphagia clinician to highlight the deviant aspects of the swallow which informs treatment. The two most commonly used instrumental procedures in the evaluation of swallowing disorders are the videofluorographic evaluation of swallowing (VFES) and the fiberoptic endoscopic evaluation of swallowing (FEES). Other instrumental approaches that may be employed in the evaluation and treatment of swallowing disorders include pharyngeal manometry, scintigraphy, electromyography (EMG), and ultrasound. The choice of instrumental approach may be dictated by the clinical question. (See Box 3.31 for comparison of popular instrumental techniques.)Regardless, the goal of the initial instrumental exam is to identify deviant aspects of the anatomy and physiology relative to swallow safety and efficiency across a variety of bolus conditions, and develop a hypothesis about the deficit such that you can generate possible treatment approaches, some of which should be trialed during the evaluation. This may be completed with a single instrumental approach or multiple approaches may be employed. While combined instrumental approaches are appealing, the current standard of care is such that most dysphagia clinicians choose either VFES or FEES for an initial evaluation (Kelly, Leslie, Beale, Payten, & Drinnan, 2006; Rao, Brady, Chaudhuri, Donzelli, & Wesling, 2003).
Box 3.31: Comparison between two commonly used instrumental exams

 Videofluorographic Evaluation of Swallowing (VFES)
Videofluorographic Evaluation of Swallowing (VFES)
The videofluorographic evaluation of swallowing (VFES) is often considered the gold standard for evaluation of swallow physiology. The procedure, which has also been referred to as modified barium study (MBS), Videofluorographic Swallow Study (VFSS), and the Oral-Pharyngeal Motility Study (OPMS), uses real-time radiographic fluoroscopy to visualize barium-laden boluses as they move through the oral and pharyngeal cavity during swallowing (Box 3.32). The data are collected as a sequence of images that can be played serially to provide movement. Due to the rapidity at which the typical swallow occurs, the resolution of the study is a function of the number of images collected per second. With older technology, this typically meant that the x-ray was run on continuous fluoroscopy and the video recorded the data at 30 frames per second. Newer digital technology allows for variation in frame rate, pulse rate, and fluoroscopy rate. While there is no clear standard for these rates, more images per second produce greater resolution. However, greater resolution comes at the cost of greater radiation exposure. Conversely, as the number of images per second is reduced, there is an increased risk of missing specific swallow deficits (Bonilha et al., 2013). Although it is possible that the risk increases as the radiation exposure decreases, the sensitivity of the exam is also reduced potentially rendering it insufficient to perform adequate evaluation.
Box 3.32 Video: VFES samples
The ultimate goal of the VFES is to identify normal and abnormal anatomy and physiology of the swallow with respect to bolus flow, biomechanical, and temporal events. Through observation of the various bolus consistencies and volumes, the dysphagia clinician identifies the aberrant physiologic events and links these to specific causes. While the procedure does not directly assess oropharyngeal sensory and motor function, these aspects are indirectly observed from noted alterations to the physiologic events. The effectiveness of bolus modification or other compensatory strategies to improve current safety of oral intake can be tested. Other recommendations and referrals may be identified as a result of the VFES.
Clinical Note
For patients who require a VFES, yet are unable to come to the fluorography suite or require a special chair for feeding that exceeds the width of the standard fluorography suite, a C-arm can be used. To learn more about this equipment and procedure, please see Davis, Palmer, & Kelsey (1990).
Clinical Note
VFES should include both lateral and AP projections. When only lateral views are recorded, the clinician may miss information on asymmetric bolus flow and pharyngeal constriction.
Procedure
The procedure as originally described by Logemann (1983), used an open field that showed the oral, pharyngeal and cervical esophagus. The radiologist would turn on the fluoroscope, which would remain on during a complete single swallow. Patients were given various consistencies and volumes to swallow. While most swallows were performed in a lateral plane, anterior-posterior projections were also used to denote asymmetrical bolus flow. This procedure became integrated into our scope of practice, and although there was similarity in the procedure across facilities, there was no universally standardized protocol.
Since then there has been advocacy for the use of a standardized procedure performed by trained and experienced clinicians. The goal to standardize the procedure has led to the development of the Modified Barium Swallowing Impairment Profile (MBSImP), which includes not only a standard procedure, but also includes a standardized analysis approach. Box 3.33 provides a sample VFES procedure inspired by the MBSImP protocol. (See Box 3.34 for an external link to the MBSimp website.) In general, various measured volumes and consistencies are presented in both lateral and anterior-posterior views. When only lateral views are recorded, the clinician may miss information on asymmetric bolus flow and pharyngeal constriction.
Box 3.33: Sample VFES procedure with instructions
LATERAL VIEW
LIQUID
- 5ml via spoon (“Hold this in your mouth until I ask you to swallow.”)
- Repeat at least twice for thin viscosity
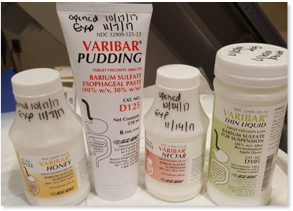
- Perform using a variety of viscosities including thin (IDDSI 0-1), nectar (IDDSI 2), honey (IDDSI 3), and pudding (IDDSI 4)
- Note that the addition of barium makes it difficult to achieve an IDDSI level 0
- Typical bolus size through controlled Single Sip (“Take a sip as you normally would, but hold it in your mouth until I ask you to swallow.”)
- Provide patient with cup that contains 30 cc
- Measure remaining liquid to calculate typical bolus size
- Sequential swallow (“Drink this in your usual manner until I tell you to stop.”)
- Provide patient with cup that contains 50 cc
- If you want to calculate swallow capacity with thin viscosity, have patient swallow the entire amount and alter instructions (“Drink this as quickly as is comfortably possible.”)
- Should be repeated with a thicker (nectar/ IDDSI 2) viscosity
SOLID
- 1⁄2 cookie coated with barium (“Chew this as you normally would and swallow when you feel comfortable and ready.”).
ANTERIOR-POSTERIOR VIEW
LIQUID
- 5ml via spoon (“Hold this in your mouth until I ask you to swallow.”)
- Repeat twice for thin viscosity
- Perform using a variety of viscosities including thin (IDDSI 0-1), nectar (IDDSI 2), honey (IDDSI 3), and pudding (IDDSI 4)
OTHER TASKS
- Assess vocal fold function (“Lift your chin and say ah.”)
Box 3.34 External Link: Link to MBSimp website
Learn more about the reasoning behind the MBSimp, as well as the components and impairment scores. See a sample evaluation report using this approach.
The equipment needed to perform this procedure is typically outside the budget of the average speech-language pathology service. Therefore, the SLP typically utilizes the equipment from the local radiology department. The dysphagia clinician may perform this procedure with help from the radiology technician or jointly with the radiologist.
The VFES may be performed after a clinical evaluation or as the initial evaluation. In the latter scenario, there may be time dedicated to history gathering and a cursory oral mechanism inspection. Other sensory and motor information will be extracted from observing the physiologic events with respect to bolus flow.
Room and bolus preparation is best completed before the patient enters the fluorography suite. This includes preparation of various bolus consistencies. Standardized viscosities can be commercially acquired in a pre-mixed format. Room preparation usually entails moving the table into an upright position. Patients can either sit on the footboard, which when the table is upright becomes a small seat or on commercially available chairs that can be used for patients with limited mobility or transfer skills. These chairs are designed to fit between the table and the image intensifier with ease.
Once the room is prepared, the patient is positioned between the upright table and the fluoroscope. The patient is given each bolus individually and instructed to swallow. While the clinician is performing the VFES, they are observing the bolus flow and generating a swallow problem list. For some populations, such as those with developmental disability, who show preference in food selection, after standardized bolus sizes and consistencies are administered, preferred foods may be mixed with barium and used during the evaluation. After bolus administration is completed, the identified swallow deficits can be used to determine appropriate compensatory strategies, which are trialed during the evaluation.
In cases where the individual is not an independent feeder, the caregiver may be asked to model a typical feeding procedure. This allows the procedure to be evaluated during the VFES. This is particularly useful when the clinician proposes a change in the feeding procedure, as the VFES can provide evidence of the benefit of the suggested change(s).
Data Analysis
Although much of the general analysis can be performed during the evaluation, the clinician often reviews the recorded exam to clarify specific data points. The key to understanding the impact of a swallow deficit is to link the videofluorographic observations to meaningful aspects of swallow function. The clinician observes the biomechanical events as the bolus flows through the oral cavity, pharyngeal cavity (while avoiding the airway entrance), and cervical esophagus. In addition, the clinician identifies the presence and degree of penetration and/or aspiration, as well as the physiologic reaction to airway invasion. After the swallow, the location and amount of bolus residue is noted as well as any additional penetration or aspiration from the residue.
Upon completion of the exam, the clinician can review the recorded exam to confirm ratings and make other physiologic measures. Temporal variables such as oral transit time, pharyngeal transit time, and UES opening duration can be measured from the VFES. Other physiologic parameters such as UES opening or hyolaryngeal trajectory can be directly measured from individual frames from the VFES. During the analysis, the clinician notes the impact of bolus volume, consistency, and other trialed compensatory strategies on swallow safety and efficiency.
Clinical Note
Temporal measures for the pharynx include:
- Pharyngeal Delay Duration (PDD)– If the bolus enters the pharynx but the pharynx does not have a timely response to the bolus. In a healthy individual, this should be zero. Also referred to as stage transition duration.
- Pharyngeal Response Duration (PRD) –The time from the onset of hyolaryngeal elevation until the tail of the bolus is through the UES.
- Pharyngeal Transit Duration (PTD)– The amount of time that the bolus is traveling through the pharynx. It is the sum of the delay time and the response duration.
Rating Physiologic Events: To perform efficient and consistent analysis, the dysphagia clinician trains in the use of rating scales such as the penetration-aspiration scale (PAS), or the MBSImP analysis of individual physiologic events. While MBSImP offers a training program, which measures clinician reliability and provides certification, facility-specific training may also occur. Box 3.35 lists physiologic events that can be evaluated with the VFES.
Box 3.35: Physiologic events that can be evaluated using VFES
- Lip closure *
- Tongue control and function *
- Bolus preparation/mastication *
- Bolus transport *$
- Initiation of pharyngeal swallow (onset of hyolaryngeal elevation) * $
- Soft palate elevation and seal *
- Hyo-laryngeal elevation (may be subdivided into maximal superior and anterior trajectory) * +
- Tongue base retraction *
- Laryngeal vestibule closure (as judged by penetrance at the height of the swallow) *
- Glottal closure
- Pharyngeal stripping wave *
- Pharyngeal contraction or obliteration * +
- Epiglottic inversion *
- UES opening * $ +
- Esophageal clearance *
Note: * items have a correlating MBSimp scale; + items have been analyzed using ImageJ, $ items can be measured temporally
In the MBSImP standardized data analysis procedure, 15 physiologic events are analyzed using a 0-3 or 0-4 point scale, where zero reflects a healthy swallow with respect to that physiologic event. (In Box 3.35 starred (*) items are components that can be scored using the MBSimp.) Using an online training program, clinicians practice scoring the various components on individual swallows.
Clinical Note
For more detail on how to perform ImageJ analysis on hyolaryngeal elevation, UES opening, or pharyngeal compression, you can check out the approaches used by the following authors.
STEELE: Dr. Steele’s lab has published extensively on kinematic analysis to assess hyo-laryngal trajectory. She maintains links to the excel spreadsheet that can be used to produce automated numbers. Steele and colleagues have also developed the Normalized Residue Ratio Scale which uses ImageJ to compare the size of the entire space versus the space with infiltrate.
VANDAELE: Dr VanDaele produced early publications on the use of digital image analysis on hyo-laryngal trajectory. You can review his methods by reading Kellen et al., 2010.
LEONARD: Dr. Leonard and colleagues discuss their technique for using digital image analysis to assess not only hyo-laryngeal trajectory, but also pharyngeal compression in their textbook titled, Dysphagia Assessment and Treatment Planning.
Using ImageJ, specific physiologic variables such as hyolaryngeal trajectory, pharyngeal compression, or UES opening, can be directly measured from still frames extracted from the VFES. For hyolaryngeal trajectory, maximal displacement from rest can be measured using both maximal superior displacement and maximal anterior displacement. The units of measurements can be compared to a known entity, such as a penny (19.05 mm) on the neck, or related to a physiologic constant, such as cervical vertebrae referred to as a cervical unit).
Rating Residue: Residue after the swallow is of interest to the dysphagia clinician as this material leaves the individual at risk for aspiration after the swallow. The primary oral locations of residue include the lateral sulci, the anterior sulci, and floor of mouth (under the tongue); the primary pharyngeal locations of residue include the valleculae space, pyriform sinuses, pharyngeal wall, along the laryngeal rim or in the larynx. In addition to residue location, dysphagia clinicians may attempt to qualify the amount of residue in each location. While oral residue can be visually inspected for amount, pharyngeal residue must be estimated from the 2-D image. Multiple approaches have been proposed for the estimation of pharyngeal residue during the VFES. The MBSImP has two residue parameters—one for oral and one for pharyngeal. A visual review of residual barium after swallow completion is judged to range from (0) none to (4) retention of the entire bolus amount.
In an attempt to produce more quantitative measurements of bolus residue, use of digital image analysis can yield a ratio of space containing residue by measuring the total space of the vallecula or pyriform sinus and comparing it to the space containing residue. More recently, Pearson and colleagues (2013) developed the Normalized Residue Ratio Scale (NRRS). This scale uses digital image analysis software (Image J) to provide a measure of the pharyngeal residue relative to the total pharyngeal space and normalized to the size of the individual. Two subscales are employed— one for the valleculae and one for the pyriform sinuses. The procedure requires training, but produces consistent findings within and across raters. The primary limitation of the scale is that it does not produce intuitive numbers. For example, if a true ratio were used, you could easily interpret a 1 to mean that the entire space was filled with invasion. This is not the case with the final output obtained from the NRRS in its current format. Further, this scale does not address the residue on the pharyngeal wall (which can be adequately measured with an ordinal scale—e.g., none, trace…), nor does it address oral residue.
Penetration and Aspiration: Box 3.36 presents the scoring system used in the penetration-aspiration scale which was developed by Rosenbek and colleagues (1996). While identification of the presence or absence of aspiration is not the goal of the VFES, it is an essential aspect of the data analysis. The Penetration-Aspiration Scale provides a comprehensive breakdown of various aspects of laryngeal penetration including depth of penetrance, response to penetrance, and success of the clearance of airway invasion. Although the 8-point scale does not seek to identify the timing of the penetrance event, an important aspect when deciding on treatment, parenthetical notations can be used to aid the clinician in this variable. Various approaches have been used to incorporate the PAS into the analysis of the VFES. A single score depicting either the worst observation (highest number on the scale) or an average of the PAS score across swallows may be used.
Box 3.36: Penetration Aspiration Scale developed by Rosenbek et al. (1996)
Score Description of Events
- Material does not enter airway
- Material enters the airway, remains above the vocal folds, and is ejected from the airway.
- Material enters the airway, remains above the vocal folds, and is not ejected from the airway.
- Material enters the airway, contacts the vocal folds, and is ejected from the airway.
- Material enters the airway, contacts the vocal folds, and is not ejected from the airway.
- Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway.
- Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort.
- Material enters the airway, passes below the vocal folds, and no effort is made to eject.
Source: Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996.
Limitations and Risks
In the past, research showed that while VFES was reliable for detection of aspiration, discrete physiologic events were not reliably identified across raters (Kuhlemeier, Yates, & Palmer, 1998; Stoeckli, Thierry, Huisman, & Martin-Harris, 2003). A more recent study clarified that reliability varied across the physiologic events where some events are rated with high agreement across clinicians and others are not (Kim et al., 2012). Across all physiologic events, inter-rater reliability was lowest on oral phase parameters, particularly premature spillage. After providing training for specific physiologic parameters and using standardized bolus sizes, reliable agreement across clinicians regarding the level of severity for each physiologic event increased to 80% across all parameters—oral and pharyngeal (Martin-Harris et al., 2008). The summary here is clear. VFES provides a clear diagnostic advantage as it allows bolus flow visualization throughout the swallow phases. Yet, the outcome of VFES is linked to training and standardization of procedures and physiologic parameters. When these are lacking, reliability is compromised.
The risk:benefit ratio associated with radiation exposure is a topic of much debate. When performing any clinical exam, the clinician must reconcile the risk:benefit ratio. In the case of VFES, the potential outcome gained from visualization of bolus flow through the swallow must outweigh the risk associated with the anticipated radiation exposure. Risks can be kept to a minimum by limiting the time of the exam. Also, pulsed rates can be used to reduce the time exposed to fluoroscopy, thereby reducing the total amount of radiation exposure. While radiation exposure occurs in the natural environment, the concern here is the increased radiation exposure associated with manmade radiation for diagnostic purposes. Medical studies that use radiation are geared to provide radiation “as low as reasonably achievable” (ALARA). Radiation exposure is linked to study duration and pulse rate. Thus, one way to keep radiation as low as possible is to limit the study time. It is typical to set a timer and terminate studies that have not been completed within an allotted time frame (e.g., 3 minutes).
Just for Fun
Watch this silly video about radiation exposure during the VFES procedure.
FEES-Fiberoptic Endoscopic Evaluation of Swallowing
The fiberoptic endoscopic evaluation of swallowing (FEES) was first described by Langmore and colleagues (1988) as a substitute for videofluorographic examination for patients who could not make it to the fluoroscopy suite or where radiation exposure was deemed to great a risk for a specific patient. They promoted inserting a standard transnasal flexible scope into the pharynx during the swallow to assess pharyngeal and laryngeal function before, during, and after the swallow (Box 3.37).
Box 3.37: FEES videos samples
Click here to watch: https://youtu.be/ohsEtgEn9KE
“Want more?”
Watch this overview of nasal anatomy.
Due to its portability, the FEES procedure can be performed in an outpatient clinic or at the patient’s bedside. The equipment needed to perform a FEES may be as a simple as a nasal endoscope and a light source. However, to provide optimal evaluation, the endoscope is connected to an external monitor for viewing, as well as recorded and stored for future review or comparison. Today endoscopes can be fiberoptic or digital chip. Scopes come in different diameters to accommodate various ages and sizes of nasal space.
Although not consistent across facilities, written consent may be obtained for the procedure due to the unlikely event of laryngospasm, nose bleed, or vasovagal response. In some facilities, verbal consent is used in place of written consent.
“Want more?”
When first introduced, FEES stood for FIBEROPTIC Endoscopic Evaluation of Swallowing. However, with the advent of chip-tip technology, the F in FEES now stands for FLEXIBLE. This name will accommodate the use of any flexible endoscope.
Procedure
Procedure preparation includes gathering desired boluses. The clinician may choose to add a colorant, such as blue dye to the various test boluses. This is performed to improve the visibility of the bolus in the pharynx. However, with current technology, the FEES procedure retains its reliability whether or not a colorant is used by the clinician (Leder, Acton, Lisitano, & Murray, 2005). Although the procedure can be performed in any position, the patient is typically sitting upright. (See Box 3.37 for sample clips from FEES.) For patients with mobility issues, this procedure allows the clinician to test the patient in the position that they usually eat. Before scope insertion the clinician determines which nasal passage will be used and if the nasal passage will be prepared with a topical anesthetic or vasoconstrictor. Choice of nostril (right versus left) may be a function of the nostril with the best patency or may be a function of the location of the equipment. A quick visualization of the nasal cavity may indicate a deviated septum or reduced airflow on one side versus the other.
While floor of the nose is the most commonly used route for FEES, at times, when velar function is also under investigation, the clinician may choose to route the scope through the middle turbinate to reduce pressure on the palate and enhance the ability to incorporate a palatal assessment into the procedure. While in route to the pharynx, the dysphagia clinician may pause the scope above the velopharyngeal valve to evaluate movement. The following stimuli may be used to note any weakness or leaks in the velopharyngeal valve during closure–speech, whistle, saliva swallow and wet swallow.
Once the velar assessment is completed, the scope is advanced to the oropharynx where the tip of the scope approximates the end of the velum at rest. This affords visualization of the complete pharynx and larynx before, during and after the swallow. Prior to assessment of swallow function, anatomical structures should be visualized at rest to note any abnormalities in shape or color. (See Box 3.38 for a sample visual inspection protocol.) A small amount of anatomical asymmetry or asymmetry with posturing may be within functional limits. However, significant asymmetry in anatomical structures needs attention and consideration. Abnormal movement at rest should be noted. You may observe the larynx during quiet respiration, sniffing, breath holding, and production of a voluntary cough. Functional analysis of laryngeal and pharyngeal movement may include speech tasks, such as repetition of “kuh”, to evaluate tongue base movement, or “uh” to evaluate glottal valving. The pharyngeal squeeze maneuver, where the patient is asked to produce the highest pitch possible, will afford estimation of pharyngeal constrictor function. Box 3.39 lists sample motor tasks that can be performed during the FEES.
Box 3.38: Sample anatomical inspection to be performed during the FEES
1. Integrity of structures at rest
-
- First nasal cavity, then pharyngeal and laryngeal cavity
- For better visualization of the pyriform sinuses, ask patient to hold breath and blow out cheeks
2. Observe glottis during inhale and exhale
3. Observe structural symmetry
4. Observe appearance of tissue
5. Note presence of lesions
6. Note presence of pooled secretions
-
- Amount and location of secretions
- Spontaneous swallow frequency as this may be linked to the pooling
- Any food residue from previous oral intake
Box 3.39: Sample assessment of motor function during FEES
1. Velopharyngeal closure
-
- Speech Tasks
- Sustain ee, ss, or other oral sounds
- Alternate oral and nasal sounds (“duh-nuh”)
- Dry swallow
- Optional: Have patient swallow thin liquid. Look for nasal leakage.
- Speech Tasks
2. Base of tongue:
-
-
- Speech Tasks
- Produce kuh kuh kuh
- Speech Tasks
-
3. Pharyngeal constrictors:
-
- Hold a high-pitched, strained ee
4. Laryngeal function
-
- Sniff, pant, or alternate ee with light inhalation while observing adduction and abduction
- Phonation
-
-
- Hold “ee” (glottic closure)
- repeat “hee-hee-hee” five to seven times (symmetry, precision)
-
-
- Laryngeal Elevation
-
- effortful pitch glide
-
- Airway protection/ Breath-hold/ Glottal Closure
- hold breath lightly (true vocal folds)
- hold breath tightly (ventricular folds; arytenoids)
- hold breath to the count of 7
- effortful cough or throat clear
There is no universally-accepted standardized protocol for assessment of swallow physiology during the FEES exam. A sample procedure inspired by the work of Langmore (2001) is included in Box 3.40. However, most facilities have an agreed-upon protocol(s) likely inspired by this Langmore protocol. Swallows of various consistencies and volumes may be utilized. Compensatory strategies can be employed and assessed for effectiveness.
Box 3.40: Sample trial swallows to be used during FEES
- Ice chips: usually one-third to one-half teaspoon, dyed green
- Vary by consistency
- Thin liquids: milk, juice, formula.
- Milk or other light-colored thin liquid is recommended for visibility.
- Thick liquids: nectar or honey consistency; milkshakes
- Puree/ Pudding
- Semisolid food: mashed potato, banana, pasta
- Soft solid food (requires some chewing): bread, soft cookie, casserole, meat loaf, cooked vegetables
- Hard, chewy, crunchy food: apple, lettuce, chips
- Mixed consistencies: soup with food bits, cereal with milk,
- Thin liquids: milk, juice, formula.
3. Vary by volume
- <5 cc if patient is medically-fragile and/or pulmonary clearance is poor
- 5 cc
- 10 cc
- 15 cc
- 20 cc
- Single swallow from cup or straw: monitored
- Single swallow from cup or straw: self-presented
- Free consecutive swallows: self-presented
- Feed self food at own rate
**Note: Consistencies will vary depending on patient age and problems observed.
Incorporation of sensory assessment into the FEES has been performed using both air puffs (FEESST) and tactile stimulation from the scope (Aviv, Martin, Keen, Debell, & Blitzer, 1993; Kaneoka, Krisciunas, Walsh, Raade, & Langmore, 2015). The FEEST as originally described by Aviv and colleagues (1993) used a channel on the flexible scope to deliver a puff of air to the supraglottic laryngeal mucosa to elicit a laryngeal adductor reflex. This procedure has not been adopted by most clinics, perhaps due to the extra equipment needed. A more intuitive approach that requires no additional equipment is to lightly touch the tissue of the aryepiglottic fold or epiglottis with the scope tip and note the response. In this procedure, laryngeal sensation is considered to be grossly intact if any of the following are noted including the laryngeal adductor reflex, a cough or throat clear, or even blinking or tearing of the eye. Comparison of the two procedures showed that while the FEEST is better at detecting mild sensory deficits, it often overestimates the presence of sensory deficits. Both methods perform well for identification of severe sensory deficits (Kaneoka et al., 2015). The results of the touch method were more closely related to the scores obtained on the PAS. More work is needed in this area to better identify and describe ways to control the pressure used during this assessment. See Box 3.41 for sample sensory testing during FEES.
Box 3.41: Sample assessment of sensory function during FEES
- Note response to presence of scope
- Lightly touch pharyngeal walls, epiglottis and aryepiglottic folds
- Note response to presence of food or liquid as it goes through the pharyngeal cavity
- Note the response to any residue after the swallow
Data Analysis
Data analysis of the FEES allows description of pharyngeal and laryngeal structures and mucosa at rest including any visible alterations to anatomy (surgical, traumatic, or congenital) Presence of tics or tremors and asymmetry can be noted. Observation regarding the moisture level of the tissue, as well as the presence of any pooled secretions are noted and may be scored using a secretion scale. (See sample secretion scale in Box 3.42.) Healthy tissue will be moist without accumulated secretions. Pooled secretions have a high correlation with aspiration risk (Murray, Langmore, Ginsberg, & Dostie, 1996). When secretions are observed, note the location and amount of secretions, as well as the level of viscosity of the secretions and if they contain particulate matter from previous meals. A formal secretion scale, such as the one proposed by Murray and colleagues (1996), may be employed for improved communication with other professionals and tracking over time.
Box 3.42: Secretions scale. of sensory function during FEES
Proposed by Murray et al. (1996), the secretion scale uses a scale from 0-3.
0 = no visible secretion
1 = secretions in the pyriform sinus, valleculae space or along the laryngeal rim
2 = rating between 1 and 3
3 = secretions penetrating past the laryngeal aditus
Structural movement can be documented with respect to range of motion during swallow and non swallow tasks (Box 3.39). Pharyngeal strength can be estimated based on the results of the pharyngeal squeeze maneuver. The glottal valving and laryngeal mobility required for efficient and safe swallowing, can be initially assessed during phonation tasks. The biomechanics of the swallow are limited to the pharyngeal phase of the swallow. However, some observations may implicate oral deficits, such as premature spillage of material into the pharynx or pumping of the base of tongue before the bolus is transported to the pharynx. Laryngeal penetration or aspiration before or after the swallow can be observed and the physiologic reaction to the event should be noted. The PAS can be used successfully with FEES to quantify penetration and aspiration events (Colodny, 2002). The FEES can also provide greater detail than the VFES on laryngeal closure with respect to timing of internal laryngeal events.
Residue: Pharyngeal residue after the swallow is easily visualized during the FEES. The clinician can note the location and amount of the residue as well as the patient’s reaction to the residue. Several residue scales have been developed. Boston Residue and Clearance Scale (BRACS), a perceptual scale, has been proposed for use with FEES (Box 3.43). This ordinal scale incorporates three aspects of residue including location and amount, reaction to the residue, and results of the reaction (Kaneoka et al., 2013). Similarly, the Yale Pharyngeal Residue Severity Rating Scale (YPRSRS) (Neubauer et al., 2015) is an ordinal scale that reports residue location and amount (Box 3.44). The primary differences between these scales are that, while the BRACS uses a scale of 0-3 (none to severe), the YPRSRS rates residue on a scale from 1-5 (none to severe). As both instruments are valid, reliable, and easy to use, it seems a matter of clinician preference.
Box 3.43: Boston Residue and Clearance Scale
Indicate the location and amount of residue. Mark all that apply. The chart below is completed for each individual bolus and the score reported is the worst score obtained in each zone across the boluses.

Scale format derived by the author based on information from Kaneoka et al. (2013) and Field (2013).
Box 3.44: Yale Pharyngeal Residue Severity Rating Scale
Using the descriptions below, identify the residue severity for the valleculae and the pyriform sinus regions.

Adapted from Neubauer et al. (2015)
Sensory Function: A gross estimate of sensory function can be obtained by observing the reaction that is elicited when the structures make contact with the scope, secretions, or residue. Reactions to any penetration or aspiration during the swallow exam can be used to further support the clinicians’ understanding of the regional sensory function. In addition to these informal observations, elicited responses can be observed with purposeful and guided scope contact with a specific structural location, as previously explained (Box 3.41).
Laryngeal Function: A closer investigation of the larynx can be performed by advancing the scope toward the laryngeal vestibule. This can be done during phonation and speech so the clinician can note vocal fold movement. Asymmetry of movement or previously undiagnosed paresis or paralysis should be noted as it may impact glottal valving during the swallow rendering airway protection impaired. Poor respiratory function may alter the respiratory-swallow interface and result in inadequate or mistimed respiratory cessation.
Limitations and Risks
The FEES is an easy to administer and readily available procedure, but it is not without limitations and risks. Limitations may include (a) the inability to observe bolus flow through through the oral cavity, (b) the potential impact of the presence of the scope including any associated discomfort, (c) the potential impact of topical anesthetic on the system, if it is used, and (d) the loss of pharyngeal visualization during the white out period.
Suiter and Moorhead (2007) evaluated the impact of the flexible scope in the pharynx on the swallow in healthy individuals across the lifespan. Using VFES, they measured duration, PAS and the number of swallows needed to clear a bolus with and without the FEES scope in place. For the parameters measured, the authors found no alteration across the two conditions. While the analysis was limited, this encouraging finding provides some initial evidence of the lack of impact of the scope on the pharyngeal swallow.
The use of topical anesthetic during a FEES varies widely across clinicians. When applied, the goal is to reduce patient discomfort during the procedure. Several points have been discussed with respect to the use of topical anesthetic. First, there is conflicting evidence regarding the change in procedure tolerability as a result of anesthesia. Second, the there is no standard for the use of or the amount of anesthesia applied during the procedure. Finally, when anesthesia is used in the nares, there is concern that it may drip into the pharynx and alter motor and sensory function related to swallowing.
Multiple studies have shown that the use of anesthetic does not significantly improve the experience for the patient (Frosh, Jayaraj, Porter, & Almeyda, 1998; Kamarunas, McCullough, Guidry, Mennemeier, & Schluterman, 2014; Leder, Ross, Briskin, & Sasaki, 1997). Yet, recent evidence suggests that patient tolerability is improved and pain is reduced with anesthesia (O’Dea et al., 2015). Concerns regarding the impact of anesthesia on pharyngeal and laryngeal motor and sensory function have also been explored. While there appears to be no impact on the laryngopharyngeal sensory thresholds (Kamarunas et al., 2014), temporal measures may be impacted by the use of anesthesia (Kamarunas et al., 2014). The impact of anesthesia may be thwarted by using controlled dosing. Specifically, 0.2mL of 4% lidocaine may provide improved procedure tolerance without impacting pharyngeal sensory or motor function (O’Dea et al., 2015).
Risks include nosebleed and a vasovagal response as a result of the presence of the scope in the nares or pharynx. The limitation that is universally accepted is that the FEES only evaluates pharyngeal events. Furthermore, even pharyngeal events are blocked from view during the white out period (a period of time when the pharynx closes around the scope). UES function is also difficult to assess with the FEES procedure. Although the oral aspects can be visually inspected, the entire bolus flow pathway is not visualized. In unhealthy individuals or individuals who lack in pharyngeal function, the scope white out period may be reduced thus having less impact on the limitations of the FEES procedure.
High Resolution Pharyngeal Manometry (HRM)
Esophageal manometry has been been used for more than a decade to measure pressure in the esophagus by inserting a manometer into the esophageal body. Solid-state technology (as opposed to water perfused manometers) has afforded application of this technique to the pharynx. Recently, the advent of high-resolution manometry has further enhanced its clinical usefulness for the evaluation of swallow events associated with pharyngeal pressure generation and UES opening (i.e., CP relaxation). Although clinical utility of this procedure is still in its infancy, ASHA denotes it as an emerging technology that will likely increase in clinical utility over the next decade, particularly for patients with complex dysphagia. This device is currently not used as the primary choice for an initial evaluation. Yet, when added to current imaging procedures (i.e., VFES), the pressure generation profiles compliment the biomechanical data and enhance diagnostic yield.
Clinical Note
From the HRM exam the SLP can obtain (a) the total swallow duration, (b) the maximal pressures associated with velopharyngeal closure and tongue base retraction, and (c) the drop in UES pressure along with the duration of the pressure drop.
Clinical Note
Catheters used for high resolution manometry can come with or withoutimpedance. When impedance is added the catheter has a thicker diameter. The benefit of impedance is that it allows you to assess bolus flow during saline swallows. More research is needed to learn about the linkage between the HRM and the VFES. A clinically useful direction would be to develop an evaluation procedure that combines and time syncs the two data images.
Described simply, a manometer tube (which vary in diameter from about 2.7 to 4.2 mm, and presence or absence of an impedance channel) is placed transnasally. While blind placement of the manometer is typical, at times, placement may be guided by endoscopic visualization. This is particularly useful when a patient has poor glottal valving during the swallow. Manometers may be adorned with impedance channels that provide further information about bolus flow, yet also increase the width of the manometer. Once the manometer is in place, the patient is asked to swallow. (See Box 3.45 for sample protocol.) When impedance channels are present, saline or salty applesauce may be used during this procedure.
Box 3.45: Sample HRM protocol
- Catheter inserted
- Sensor locations confirmed and the catheter position stabilized
- Presentation of measured saline boluses
- 1ml, 5ml, 10ml, 20ml
- Trial compensatory strategies as indicated
Once the data are obtained, the pharyngeal pressure profiles are evaluated for both maximal pressure generation and pressure duration. (A typical HRM plot is provided in Box 3.46.) Maximal pressure can be measured for the nasopharynx during velopharyngeal closure, tongue base retraction, and UES post swallow pressure. Duration of pressure events can yield a total swallow duration and UES open duration. These data, especially when combined with VFES, are particularly useful in aiding in differential diagnosis of UES dysfunction (Knigge, Thibeault & McCullouch, 2014).
Box 3.46 Video: High resolution manometry plot overview
For images in this movie: No machine-readable author provided. Skg135~commonswiki assumed (based on copyright claims). [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
This procedure is currently under-utilized with limited peer-reviewed publications available. The procedure has some similarity to a FEES and therefore has some of the same risks associated with scope passage. The information obtained reflects pharyngeal pressures only. The clinician requires training and practice to perform the procedure and correctly extract the pertinent pressure data. As the manometer is typically larger than the standard endoscope, patients may experience discomfort associated with passage even with the use of anesthesia.
“Want more?”
If you want to learn more about high resolution manometry,you can view these two videos.
Laborie Medical (makers of the instrumentation) provide a summary of the theory and technology.
Ultrasound
Ultrasound is a method used to evaluate soft tissue. The technology involves the use of sound waves to generate an image whose contrast is provided by differences in each tissue’s ability to reflect sound. Using a surface transducer placed on the skin in the region of interest, sound waves are delivered to the tissue and are reflected back when there is a boundary interface, such as tissue and air, or tissue and bone. The reflection coefficients allow for real-time reconstruction of the soft tissue in the region of the transducer. During swallowing, the movement of the soft tissue required to accommodate the bolus flow allows for visualization of the swallow.
Ultrasound has been used to visualize swallowing in infants and adults. Yet, this procedure is currently not widely used as a clinical tool. It is plausible that as the 3-D technologies become more readily available to clinicians, ultrasound will be used with greater frequency, particularly in the infant population.
The primary limitation to the use of ultrasound as a diagnostic tool for swallowing is the inability of ultrasound to penetrate bone. That means that if a bone is present, then anything behind that bone is in a shadow (i.e., not visualized). For swallow function, the mandible and hyoid bones cause a shadow and information behind those bones is eliminated from the image. The procedure is relatively free of side effects and discomfort, with the possible exception of minor discomfort from the transducer on the neck, especially in infants with tactile aversions.

