Compensatory Treatment
Compensatory treatment approaches provide immediate and transient alteration to the swallow physiology or bolus flow dynamics, rendering a safer and/or more efficient swallow. In many cases, compensatory strategies can be accomplished without direct participation from the individual with dysphagia. In general, these strategies can be divided into: (1) diet or bolus modifications, (2) postures and positions, (3) maneuvers, (4) sensory enhancement, or (5) other changes to mealtime presentation or environment. The goal of compensatory strategies is to immediately reduce the impact of the swallow impairment allowing an individual to continue to have oral intake without medical risk. The key distinction of a compensatory strategy (as compared to a rehabilitative approach) is that the swallow deficit is still present. The transient masking of the deficit is a result of the compensatory strategy being employed.
Diet Modification
Diet modifications refer to any changes in the type of food and liquids that are consumed and may include mention of viscosity or consistency, food size or bolus size, or texture of the liquid or food choices. The clinical goal of diet modification is to identify the least restrictive types of foods and liquids that can be safely and efficiently swallowed during mealtime. Alterations to the diet type impact the bolus flow with respect to speed or cohesion, or enhances the sensory input received during mealtimes.
Changes to diet choice or consistency should result from the evidence gained in the evaluation. When a diet modification is used to treat dysphagia, the modification should be the least restrictive diet that a person can safely handle. To ensure that the diet remains the least restrictive, the chosen diet should be continually evaluated and adjusted to reflect the patient’s current swallow function and nutritional needs. For most patient populations, diet modifications are considered a short-term or last resort approach to dysphagia management. Although rare, there are cases where diet modification is the primary approach, and is used long-term. For example, in patients with dementia or nearing end of life, diet modification may be the primary or only treatment.
Liquids can be modified for level of viscosity, which impacts bolus flow in predictable ways. While thin liquids move more easily in response to gravity and pressure gradients, they require coordination and control. In patients with strictures or muscle weakness, thinner liquids may be selected. Thicker liquids move more slowly in response to gravity and require a greater pressure gradient to encourage change in the bolus pathway. Thus, thicker liquids may require more strength to move at an adequate speed. When this is not provided, thicker liquids may result in increased pharyngeal residue. Thicker liquids provide greater sensory input and slow the flow rate to allow time for adequate airway protection.
Clinical Note
Impact of increasing viscosity on swallow physiology:
- More viscous material may increase pharyngeal transit time (Nascimento, Cassiani, Santos, & Dantas, 2015), and increased hyo-laryngeal trajectory (Nagy, Molfenter, Peladeau-Pigeon, Stokely, & Steele,2015).
- Increased propulsive forces and clearing pressure are required with increased viscosity (Pouderoux & Kahrilas, 1995; Rant, McKee, & Johnson, 2001).
To alter liquid thickness or viscosity, thickening agents are mixed with a base liquid, such as water or juice. Thickening agents vary in the presentation (powder or gel), as well as the thickening agent used (gum or starch). Regardless, many report that the texture or taste associated with thickening is unpleasant. Further, without strict adherence to instructions, consistent thickening is difficult to achieve. Pre-thickened products are available for purchase and provide a more consistent viscosity over time. The desired end result is to maintain the prescribed consistency for the duration of a mealtime.
Diet Terminology
While many facilities have their own terminology for diet types, there is a movement toward an internationally accepted terminology for defining diets. Currently, there are two formats that are in wide-spread use — National Dysphagia Diet (NDD) and the International Dysphagia Diet Standardization Initiative (IDDSI). Box 4.14 and Box 4.15 provide examples of each of these diets.
Box 4.14: Diet levels proposed by the National Dysphagia Diet Task Force

Box 4.15: Diet levels proposed by IDDSI
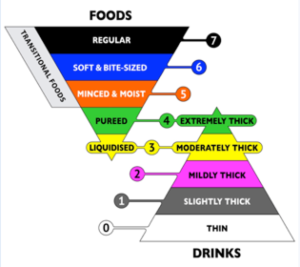
The NDD was developed by the American Dietetic Association (2002) and provided one of the first large scale attempts at the development of a framework for classification of solid foods. They identified four levels of textures (described in Box 4.14) which correspond to various levels of oral motor function. For liquids, the NDD task force has promoted the terms thin, nectar-thick, and honey-thick, clarifying the viscosity for each classification in centipoise (a standardized measure of viscosity).
More recently, the IDDSI has focused on the development of an international standard for food and liquid descriptions (Cichero et al., 2017). Here, seven levels of liquid and food thickness are described ranging from thin liquid to a regular diet. While many dysphagia clinicians are moving to the IDDSI where there are clear and easy guidelines for measuring the thickness of a liquid or food, the NDD is still used with some frequency in institutional kitchens.
Clinical Note
In general, thicker viscosities (a) have a slower flow rate than thinner consistencies, (b) provide more sensory input than thinner consistencies, and (c) invigorate some kinematic events such as epiglottic inversion. Conversely, a thicker bolus may be more likely retained in a weak pharynx than thinner liquids.
To Thicken or Not
Thickening of liquids has been the topic of much debate. Historically, for individuals who aspirated on thin liquids, thickened liquids were employed to improve swallow safety. The hypothesis is that the thickened liquids slow down the bolus flow and allow more time for neurologic awareness of the bolus, and intentionality of the bolus pathway, especially when combined with postures or maneuvers. The end result is a reduction in aspiration of liquids before and during the swallow. However, thickened liquids may require greater driving pressure to get the entire bolus through the pharynx. Therefore, thickened liquids may result in increased pharyngeal residue, which increases the risk of aspiration after the swallow. Further, if thickened liquids are aspirated, they are more difficult to eject from the airway, especially when the thickener is starch-based (Robbins et al., 2008). Thus, there is a balance needed between thickening for airway protection and thinning for improved pharyngeal clearance. Yet, there is limited data on how much thickening is required to reduce the risk of aspiration without increasing the risk of pharyngeal residue. It is likely that this value, if identified, cannot be applied to all individuals with dysphagia. Parameters related to ideal thickness for optimal swallow safety and performance may be best addressed individually under visualization, such as FEES or VFES.
Current evidence supporting the use of thickened liquids as a way to reduce the risk of aspiration is inconclusive and conflicting. Across a variety of patient groups, thickened liquids reduced the incidence of aspiration and the risk of developing aspiration pneumonia (Logemann et al., 2008; Bhattacharyya, Kotz, & Shapiro, 2003; Clavé et al., 2006). Yet, when comparing thickened liquids to postures, the incidence of aspiration pneumonia was slightly higher in patients who received the thickened liquids (Robbins et al., 2008). Notably, when postures can be successfully employed, they may be preferable to thickened liquids.
In summary, the efficacy of thickened liquids cannot be broadly applied across patients and may vary by swallow deficit(s) and medical status. Even if immediate benefits are observed during visualization of the swallow, the choice to thicken liquids (versus the use of other treatment approaches, such as postures) may not alter the risk of aspiration pneumonia. Perhaps the best support for choosing thickened liquids is to gain direct evidence of its effectiveness for a specific individual. Of course, identifying the right consistency does not imply that your clinical work is complete. You must consider patient or caregiver ability to replicate the desired thickness, as well as the patient and caregiver’s desire to adhere with the recommendation(s). If either of these are lacking, then thickening may not be the ideal choice for your patient.
“Want more?”
How do we define thickness?
Thickness of a liquid is defined by viscosity, which physically is the resistance of a fluid to deformation or a liquids internal resistance to flow. In simple terms, if you spilled a specific liquid on a flat surface, how quickly would it take for the liquid to reach the end of the surface and drip on to the floor?
The unit of viscosity is centipoise (cP); water is about 1 cP, whereas honey is about 2000 cP. Centipoise can be measured using an expensive viscometer. However, low technology approaches are more useful for the average clinician. These approaches generally include the measured flow through a syringe or funnel, or the measured spread of liquid onto a surface for a given amount of time. The commonly used line spread test, is completed by releasing a defined amount of a consistency on a sheet of concentric circles and observe the spread of the liquid on the concentric circles after one minute. This is the method that was employed in the development of the NDD. Another method used is the syringe flow method where you observe how many ccs of liquid leave an inverted syringe in 10 seconds. This is the method encouraged by the IDDSI.
Using these low technology approaches, facility specific terminology can be assessed for differences in thickness. Further, patients and caregivers can be trained in the replication of a desired thickness by using these methods to check the thickness of a material.
Types of Thickeners
Pre-thickened liquids may be purchased, or liquids can be thickened by adding a thickening agent to a base liquid. Commercial thickening products typically include one of the following thickening agents: corn starch (e.g., maltodextrin) or other modified food starch, xanthan gum, cellulose gum, or some combination of a gum and starch-based thickener.
Gum and starch thickeners behave differently as a function of the base liquid and its temperature, as well as the time period from when the thickener was added to the liquid to the time the liquid was consumed. For example, orange juice or other acidic liquids react differently when thickened with a starch-based thickener versus a gum-based thickener (Garcia, Chambers, Matta, & Clark, 2005). In general, there is an inverse relationship between temperature and thickness. A colder liquid is likely to produce a thicker liquid given the same preparation. However, this relationship is not perfect and varies depending on the base liquid.
The length of time that the liquid is standing after the thickening agent has been applied may impact the consistency of the end product. Some thickening agents may thicken over time, while others may become thinner. In general, gum-based thickeners may have better stability in maintaining thickness over time (Garcia et al., 2005).
Solids are typically not thickened, but thickness or consistency may be obtained by food selection. That is, some foods have more thickness and cohesion than others. Consider, for example, oatmeal versus ground meat.
Clinical Note
If greater pressures and muscle strength are required to move a thicker bolus, then bolus viscosity can be used as a rehabilitative approach to improve swallow function over time.
Limitations
Various concerns have been identified when choosing thickening as a primary treatment approach to reduce the risk of aspiration. These include volume of water intake, the ability to replicate the recommended consistency, adherence, hydration, gut absorption, feeling of being full, constipation, and necrotizing enterocolitis.
Reproducibility
In order to say that a given patient’s swallow safety is improved as a result of a defined thickness, patients and caregivers must be able to consistently reproduce the desired viscosity. Patients or caregivers who are not adequately trained in meal preparation may adhere with the recommendation, but provide an incorrect or inconsistent thickness at each meal. The variation in the consistency may impact swallow safety.
Even when the correct thickness is initially achieved for each meal, it may not be maintained over the mealtime. Thickness stability depends on the type of thickener used. Gum-based thickeners are more difficult to mix than starch-based thickeners and may clump over time. Starch-based thickeners may thicken liquids too much if they are prepared before mealtime and left standing for a period of time before being consumed.
Adherence
Adherence may be the biggest hurdle in the use of thickened liquids. Reduced adherence can lead to either the intake of unthickened liquids or the reduction of liquid intake. An individual’s adherence to replacing thin liquids with thickened liquids may vary for a variety of reasons. Thickening liquids changes the drinking experience by altering the texture and taste of the liquid (Matta, Chambers, Mertz-Garcia, & McGowan Helverson, 2006). If thickening the liquid renders it undesirable, it may be avoided. In fact, individuals who are provided only thickened liquids, consume less liquid then they did when their liquids were unthickened (Garon et al., 1997; Vivanti, Campbell, Suter, Hannan-Jones, & Hulcombe, 2009). Regardless, poor adherence may lead to adverse events, such as dehydration if liquid intake is reduced, or aspiration pneumonia if thin liquids are consumed (Low, Wyles, Wilkinson & Sainsbury, 2001). Further, thickeners alter the drinking experience and may impact the taste of the liquid. These changes in taste and viscosity may reduce an individual’s willingness to comply with the recommendation for liquid thickening.
Adherence to a recommendation to thicken is likely to improve with adequate education regarding: (1) the swallow problem, (2) the associated risks if the recommendations are not followed, and (3) benefits of the recommendations. Consider culturally sensitive communication preferences and include appropriate caregivers. Caregivers should receive education to aid in their support of the recommendations and their application.
Clinical Note
If a clinician honors the patient-focused ICF model the concerns around adherence are reduced. In the ICF model, the patient and caregiver(s) are provided with the information they need to decide on their treatment of choice. The patient can choose not to accept a recommendation of thickened liquids.
Oral Intake Volume
Closely linked to the adherence is the volume of liquid intake. There are data to support that thickened liquids reduce the volume of liquid intake (Garon et al., 1997; Vivanti et al., 2009). There are several possible reasons: slowed liquid flow, reduced palatability, and reduced liquid availability. Reduced volume may be a direct result of thickening due to the slow flow of thickened liquids. If a patient has 30 minutes for a mealtime, yet the liquid material is thickened and slowed as it moves through the system, it is possible that the amount of liquid and food intake may be reduced given a similar mealtime duration.
As discussed earlier, thickened liquids may be undesirable due to changes in texture or taste. Thus, liquid intake may be reduced if an individual dislikes the taste or texture of the thickened liquid. Also, thickened liquids may not produce the sensation of having your thirst quenched.
Although not directly related to thickening, for patients who are hospitalized or reside in a long-term care facility, the need for thickened liquids may translate to unavailability of liquids between meals. If thickened liquids are left at bedside, over time the thickness changes increasing the possibility that the taste or texture will discourage intake. Starch-based thickeners may become more gelatinous with time, especially when added to hot beverages. (Think about how you thicken gravy—add starch and heat).
In the past, there was concern about alterations in the bioavailability of water when thickening agents were applied. This stemmed from the observation that individuals using thickened liquids as part of their treatment for dysphagia were more likely to be dehydrated (Leibovitz et al., 2007). Although the data are limited, there is currently no evidence to support reduced bioavailability of water molecules in thickened liquid regardless of the thickening agent or the level of thickness (Sharpe, Ward, Cichero, Sopade, & Halley, 2007; Hill, Dodrill, Bluck, & Davies, 2010). The conclusion here is that individuals required to drink thickened liquids consume less, and therefore, are more prone to dehydration. In addition to reduced liquid intake, thickened liquids may result in a feeling of being full leading to reduced caloric consumption during a meal.
Clinical Note
If by thickening liquids, patients avoid liquids, there is an increased risk of dehydration. If a patient decides not to adopt the recommendation of thickened liquids, he/she can be educated about the consequences, as well as provided alternatives to thickened liquids. This conversation should be documented in the medical chart.
Gut Absorption
While bioavailability of water is not greatly reduced by thickening liquids, there is evidence that absorption of some medications may be altered when swallowed with a thickened liquid. Medications are developed with a desired absorption site and time. When thickened liquids are used the medication may not break down as intended, and therefore optimal absorption may be altered. Of particular concern are medications that should be absorbed in the upper GI region. When administered with gum-thickened water, the medication may be bound in the liquid until it reaches the lower GI system. Data are lacking on the impact of using thickened liquids to swallow medications.
Some thickeners are contraindicated in patients with (a) immature guts, such as preterm infants, (b) food allergies, or (c) guts prone to constipation. There is evidence that gum-based thickeners can result in necrotizing enterocolitis in infants. In fact, there are over 20 deaths reported in the literature that are linked to the use of thickened liquids in preterm infants (Woods, Oliver, Lewis & Young, 2012; Clarke & Robinson, 2004; Beal, Silverman, Bellant, Young & Klontz, 2012). Therefore, all xanthum gum-based thickeners should be avoided in infants. Elderly individuals may become constipated with thickened liquids, particularly starch-based thickeners. This binding can have multiple effects on the overall health and energy of an elderly individual.
Diet Modification for Solids
Diet modification is not limited to liquid boluses. Solid foods may be altered to accommodate various levels of dysphagia. Solid foods can vary in texture from liquified (IDDSI=3) to regular (IDDSI=7). Box 4.16 displays the solid textures described in the IDDSI framework. Due to the complex oral management, mixed consistencies, which include foods that contain more than 1 texture, such as a matzah-ball soup or fruit cocktail in juice, are often avoided in patients with dysphagia.
Box 4.16: Levels of solid food textures per the IDDSI

Postures & Positions
Postures are compensatory approaches that improve swallow safety by using gravity to redirect bolus flow through the swallow conduit. Changing head flexion, extension or rotation during swallowing manipulates the impact of gravity on bolus flow, as well as altering the juxtaposition of important anatomical oral, pharyngeal or laryngeal structures. Typically, treatment involves teaching the patient (or caregiver) to position the body, head, and neck in a specific way before the swallow, with maintenance of that position until the swallow is completed.
When recommending postural compensatory strategies, it is important to consider the patient’s cognitive abilities (i.e., are they able to follow instructions to learn the posture and apply the posture throughout mealtimes) and motor functions (i.e., are they physically capable of a posture). Caregivers may be employed to provide verbal and physical supports to carry out the posture. Patients with fragile instability, or structural cervical supports, such as halo or neck brace, may not be ideal for postural approaches. Individuals with altered trunk support, such as hypotonia or spasticity, may require additional assistance to apply postural compensations to swallowing. These are often best achieved by working jointly with the physical therapist.
Postures are typically considered a short-term treatment approach that will provide improved swallow safety or efficiency until the individual has experienced gain from the restorative treatment. If for reasons of cognition or other medical considerations, it is deemed appropriate to use postures as a long-term solution, some consideration must be given to changes in muscle physiology from non-use atrophy and aging. Therefore, follow up should be performed to ensure that the identified posture is still required and continues to result in a safer swallow. Currently, there are no data to help define a follow up timeline. However, changes in general health status, lung function, swallow function, or nutrition should trigger a follow up.
The effect of each posture must be evaluated for an individual with consideration of their mealtime demands with respect to volume, viscosity, and texture. Patient-specific anatomy and physiology may alter the impact of a posture on their specific swallow. This is further complicated by the patient’s ability to consistently and completely achieve the desired posture needed to get the full benefit (Nagaya, Kachi,Yamada, & Sumi, 2004). At times postures may be combined to address multiple problems. For example, a chin tuck with a head turn may be employed to increase epiglottic deflection thereby narrowing the entrance to the laryngeal vestibule, while increasing vocal fold approximation.
Chin Up
The chin up posture engages gravity to encourage efficient posterior movement of the bolus through the oral cavity, and may increase the rate of flow of the bolus entering into the pharynx (Box 4.17). This is useful for individuals lacking in oral strength or structure who are unable to generate adequate lingual pressures during the horizontal bolus trajectory. It may also aid patients with reduced tongue base to posterior pharyngeal wall contact who are unable to impart a forceful impetus onto the bolus as it begins its vertical trajectory. While chin up allows gravity to positively assist in the posterior and inferior flow of the bolus, this position also increases airway opening. Therefore, this position is reserved for individuals with adequate airway protection.
Box 4.17: Chin up posture
Sample Instructions
- Take a sip. Hold it in your mouth. Tilt your head back like you are looking up at the sky and swallow.
Impact on Swallow
- Narrows the pharynx
- Reduces vallecula space before swallow trigger
- Enlarges airway entrance
Uses
- Transport oral bolus from anterior to posterior oral cavity
- Facilitates oral to pharyngeal transport
Concerns
- Reduced vallecula space combined with greater airway opening increases the risk of aspiration
Consider using as trial during instrumental exam if you see any of the following:
- Poor tongue function or inefficient anterior-posterior oral transit with GOOD airway protection
- Reduced anterior containment
- Poor emptying of oral cavity or oral residue
Do NOT consider using if you see the following:
- Poor airway protection before the swallow
- Poor posterior containment leading to premature spillage
- Significant cervical spine dysfunction
While efficacy data is limited, using a nonrandomized controlled study, Ertekin and colleagues (2001) showed reduced dysphagia using a chin-tuck posture in patients with bilateral deficits. They also noted that the chin up posture improved swallow function in healthy individuals without dysphagia.
Chin Tuck
The most commonly used posture is a chin tuck posture (a.k.a. chin down or chin to chest) (Box 4.18). This position results in: (1) closer approximation of the tongue base and posterior pharyngeal wall, and (2) reduces airway opening while widening the vallecula space. The result makes this posture useful for patients with poor tongue base retraction or poor airway protection. Further, in cases of premature spillage or delayed swallow trigger, the widened vallecula space supports may improve protection before the swallow.
Box 4.18: Chin tuck posture
Sample Instructions
- Take a sip. Hold it in your mouth. Tilt your chin down to your chest and swallow.
Impact on Swallow
- Pushes the tongue base, epiglottis, and anterior pharyngeal wall posteriorly, thereby narrowing the airway entrance and widening the vallecula
Uses
- Protect airway
- Provide extra time for swallow to trigger
Concerns
- Chin tuck requires greater anterior oral bolus control and may require increased strength for oral anterior-posterior transport
Consider using as trial during instrumental exam if you see any of the following:
- Premature spillage
- Vallecula residue
- Delayed trigger onset
- Poor tongue base retraction
- Reduced airway protection especially before the swallow
- Poor UES opening
Do NOT consider using if you see the following:
- Poor anterior containment
- Poor posterior containment leading to premature spillage
- Significant cervical spine degeneration or dysfunction
Efficacy of the chin tuck has been shown to be useful at reducing the risk of aspiration in patients status post stroke, TBI, or esophagectomy (Terre & Mearin, 2012; Lewin, Hebert, Putnam, & DuBrow 2001; Shanahan, Logemann, Rademaker, Pauloski, & Kahrilas, 1993; Saconato, Chiari, Lederman, & Goncalves, 2016). Although, the literature does not clarify the type of aspiration (before, during or after the swallow) best treated by this posture, it has been noted that the posture does lead to increased UES open duration (Balou et al., 2014). Nor does it tease out the impact of bolus type. That is, perhaps this technique works best with liquid bolus but has reduced utility in other bolus types. These unknowns support the need for careful consideration when choosing this posture for your patient.
In addition to reducing aspiration risk, the chin tuck posture has been hypothesized to increase pharyngeal compression, and thereby, increase intrabolus pressure and reduce pharyngeal retention (Bulow, Olsson & Ekberg, 2001; Bulow, Olsson & Ekberg, 2002). There is also some evidence that this posture increases lingual pressure (Hori et al., 2011), and decreases UES pressure (McCulloch, Hoffman, & Ciucci, 2010). This combined information suggests that chin tuck may be useful in individuals with reduced oral pressure or isolated pyriform sinus residue. Yet, data that explored retention in the inferior pharynx are not supportive of this statement (Bulow et al., 2002).
Head Rotation
Head rotation (a.k.a. head turn) can be engaged for unilateral damage or poor pharyngeal pressure. In the case of unilateral damage, the head is turned in the direction of the weaker or damaged side, thereby squeezing the weakened side and encouraging the bolus to tract down the stronger side. This is a particularly useful technique in patients who have undergone surgery to the oropharynx, where the surgical procedure was largely limited to one side.
Head turn has also been used in patients with an incompetent pharynx and reduced UES opening. The action results in mechanical traction that pulls the cricoid cartilage away from the posterior pharyngeal wall and compresses the weak side of the pharynx. This increases the UES opening diameter and decreases its resting pressure (McCulloch et al., 2010). Therefore, in patients with poor UES opening or isolated pyriform sinus residue, this may be a useful position.
Low levels of evidence support the use of this posture for improving oral efficiency while reducing aspiration (Logemann, Kahrilas, Kobara, & Vakil, 1989; Ralsey et al., 1993).
Head Tilt
It is postulated that for individuals with unilateral oropharyngeal deficits, tilting the head in the direction away from the side of damage, engages gravity to bias the flow of the bolus toward the stronger side. While there is limited evidence to support or negate its use, this posture has been linked to increases in pharyngeal pressure as measured with manometry (Kim et al., 2015). Ideally, the choice to use this posture should be supported with visual imaging such as VFES or FEES.
Reclined or Side-Lying
In cases where dysphagia is significant and the individual has no success from other compensatory approaches, or is unable to follow instructions or maintain other postural choices, a reclined or side-lying position may be used. When bolus propulsion or airway protection is severely compromised, a reclined position will bias the bolus to track along the posterior pharyngeal wall away from the airway entrance. When coupled with small bolus sizes or multiple swallows per bolus, this technique, while slow, may improve safety of bolus transit, and bolus clearance. When deficits are unilateral, the patient may be slightly turned onto the stronger side.
When reclined or side lying positions are used, bolus type and bolus presentation are altered to include a cohesive bolus that may not require excessive mastication. Liquid presentation may include use of a straw with a forward head tilt, or spoon, to get liquid into the mouth. After the bolus presentation, ample time is given for the bolus to trickle down the posterior pharyngeal wall. The patient may be instructed to swallow multiple times per bolus, and cough after each bolus presentation, to clear any misdirected bolus from the airway entrance. The individual with severe dysphagia should maintain the position for a period of time after the meal is completed to obtain maximal pharyngeal clearance.
There is limited evidence to support the use of swallowing in a reclined position to improve swallow function and safety in patients with cerebral palsy (Morton, Bonas, Fourie, & Minford, 1993), muscular dystrophy (Umemoto et al., 2012), or severe dysphagia from other causes (Ota, Saitoh, Kagaya, Sonoda, & Shibata, 2011; Park, Sao, Ko, & Park, 2013).
Maneuvers
Compensatory maneuvers transiently manipulate swallow physiology through the use of a specific strategy (Box 4.19). Like positions, maneuvers should not be considered a long-term solution to swallow deficits. Maneuvers can be applied to immediately improve swallow efficiency and safety while rehabilitative approaches are being taught and used by the patient. It is possible that over time consistent use of some maneuvers may result in a long-term alteration to swallow function. In fact, there is preliminary research being done to explore the use of some compensatory strategies as rehabilitative, namely the effortful swallow and the Mendelsohn maneuver.
Box 4.19: Uses for compensatory maneuvers

Mendelsohn Maneuver
The Mendelsohn maneuver (MM), first described by Mendelsohn and McConnel (1987), employs a purposeful and prolonged laryngeal elevation during the pharyngeal swallow through voluntary active engagement of suprahyoid muscles (Box 4.20). Theoretically, voluntary augmentation of hyo-laryngeal elevation can be achieved with suprahyoid muscular engagement. By extending the superior-anterior movement of the hyo-laryngeal complex during swallowing, either with respect to height (superior and/or anterior) or duration, the UES opening will be positively altered, thereby reducing pyriform sinus pooling (Kahrilias et al., 1991; Hoffman et al., 2012). If this maneuver results in reduced pharyngeal residue, then the risk of aspiration after the swallow is also reduced. This action of purposeful prolongation of hyo-laryngeal elevation may also improve coordination and timing of swallow events (Sapienza, 2008).
Box 4.20: Mendelsohn maneuver summary
*Note: This maneuver should first be practiced without food or liquid. Between practice trials, patient may need some oral moistening. This can be achieved with a sip of water, or wiping the oral structures with a damp toothette or swab.
Initial Training:
- With the patient’s hand lightly on the your neck, ask the patient to feel the upward movement while you swallow.
- Then have the patient palpate their own neck and note the upward movement.
- Explain that this procedure will hold the “adam’s apple” up longer. With the patient’s hand lightly on the your neck, swallow and hold up the larynx.
- “Swallow and when you feel the Adam’s apple get to its highest spot, keep it there using the muscles of your mouth and neck.” You may need to alter the instructions regarding which muscles to use to get the best response.
- Once the patient is able to hold up the larynx at the peak of the swallow, work on increasing the duration of the hold with a goal of holding up the larynx for 5 seconds.
Trial Feeding or Mealtime Instructions:
- Take a small sip of liquid (bite of food).
- Swallow and when the Adam’s apple reaches its highest point, hold it there for ___ seconds.
- Release
- Repeat
While the overall spirit of the maneuver is well described in the literature, there are no standardized procedures or protocols used to teach or perform this technique. Typically the individual is educated about the action of hyo-laryngeal elevation and then encouraged to engage muscles when the hyo-laryngeal complex is at its peak. Enhancement of hyo-laryngeal trajectory may also be achieved with the use of digital assist (supporting the upward movement with the fingers) on the thyroid cartilage, or a combination of these two (effortful and prolonged muscle contraction with digital assist). Hyo-laryngeal elevation may be further enhanced by an effortful anterior tongue press. Biofeedback may be employed to improve learning and performance of the maneuver.
It is unclear which patient populations are best suited for this maneuver. As this maneuver may be difficult to understand and perform, it is best reserved for individuals with adequate cognitive function. Patients should have sufficient muscular ability (particularly in the floor of the mouth and suprahyoid muscles) as the procedure requires increased muscular effort.
With optimal application, the outcome of the MM may support: (1) a greater duration (and perhaps extent) of UES opening reducing pyriform sinus residue, (2) improved airway protection while the bolus traverses the pharynx, and (3) improved coordination or timing of the pharyngeal swallow (Sapienza, 2008).
Clinical Note
In patients post stroke, when extent or duration of hyo-laryngeal excursion is impaired, use of the MM may improve airway protection during the swallow (Pearson, Hindson, Langmore, & Zumwait, 2013).
Clinical Note
The MM is used for individuals with strokes, oropharyngeal cancer, and other neurologic disease who have reduced laryngeal elevation capabilities.As hyo-laryngeal trajectory is linked to UES opening, it is also a useful maneuver for individuals with decreased UES opening or pyriform sinus residue.
Effortful Swallow
The modified Valsalva maneuver or effortful swallow is a relatively safe and non-invasive approach that was originally employed as a compensatory maneuver to provide a temporary increase on the driving pressure of the bolus, thereby improving the transit through the oropharynx (Pouderoux & Kahrilas, 1995) (Box 4.21). As the name implies, the effortful swallow maneuver is achieved through the addition of extra volitional muscular effort during the swallow.
Box 4.21: Effortful swallow summary
*Note: This maneuver should first be practiced without saliva swallows. Between practice trials, patient may need some oral moistening. This can be achieved with a sip of water, or wiping the oral structures with a damp toothette or swab.
Trial Feeding or Mealtime Instructions:
- Take a small sip of liquid (bite of food).
- Swallow as hard as you can using all the muscles in your mouth and neck.
-
-
-
- Some authors suggest encouraging tongue to palate press when producing an effortful swallow.
-
-
The theory behind the effortful swallow is simple. Swallow is a pressure-driven event that is manipulated by the contraction of multiple oral and pharyngeal muscles. When a weak swallow is employed, speed and efficiency of bolus propulsion is reduced. This can result in slowed transit times through the pharynx with resulting distributed pharyngeal residue. Swallowing hard or with increased effort may engage more muscles or the same muscles to a greater degree than a normal swallow. When employed, the effortful swallow may improve tongue base retraction, increase oropharyngeal swallow pressure, increase hyo-laryngeal trajectory, and engage greater UES opening. It has also been shown to improve airway protection (Jang, Leigh, Seo, Han & Oh, 2015), and in healthy adults, increase peristaltic amplitudes within the distal smooth muscle region of the esophagus.
Clinical Note
The dysphagia clinician can use functional reserve to identify the ability to which a person can increase effort during swallow. For this task, functional reserve can be calculated by subtracting the average swallow pressure from maximal intraoral pressure generation. This indicates the amount of pressure available to be added to the swallow to increase effort. If swallow pressure closely approximates maximal pressure, then there is no further muscle function to be applied to the swallow. That is, an effortful swallow is already being employed. Typically, swallow pressure is approximately 40-50% of maximal pressure. Effortful swallow can employ 70-80% of maximal pressure.
For individuals who have adequate cognition to comprehend and follow instructions, along with a weak swallow, the effortful swallow may improve swallow function and bolus clearance (Huckabee, Butler, Barclay, & Jit, 2005; Witte, Huckabee, Doeltgen, Gumbley, & Robb, 2008). One way to assess a patient’s ability to employ the effortful swallow is to compare maximal intraoral force production to the force used for an individual swallow. For patients who have adequate functional reserve, greater effort can be applied during the swallow. Another approach is to observe pharyngeal compression during visualization of the pharyngeal swallow either through FEES, VFES, or HRM. Box 4.22 lists potential estimates of pharyngeal compression. When these changes are not observed, an effortful swallow maneuver can be employed to see if greater pharyngeal compression is achieved.
Box 4.22: Estimates of pharyngeal compression
- VFES
- observe pharyngeal obliteration (reduction of the white space)
- measure pharyngeal compression ratio (PCR)
- FEES
- duration of white out
- changes in pharyngeal volume
- HRM
- changes in pharyngeal pressure
The effortful swallow is helpful in improving swallow function in patients who lack strength. However, it may not be the ideal approach for patients who fatigue quickly or have diseases that make them prone to muscle injury when near-maximal force is engaged. Also, effortful swallow may be a difficult task for patients with poor saliva production. For these individuals, it may be useful to mist the mouth to improve ability to perform an effortful swallow.
As with most maneuvers, there is a training phase that requires instruction and modeling. Visual cues and level of instruction is a function of patient’s cognitive level and comprehension skills. Biofeedback may be employed to improve response and may include either submental EMG (Hiss & Huckabee, 2005; Huckabee & Steele, 2006) or lingual pressure (Sprouls et al., 2013). Although effortful swallow can be practiced without food, or with an artificial bolus, such as a oral pressure bulb, when used as a compensatory strategy, it is typically applied during oral intake and throughout mealtimes.
Effortful swallow has been used with success in healthy aging individuals (Hind et al., 2001), and individuals with dysphagia due to stroke (Robbins et al., 2007; Park, Kim, Oh, & Lee, 2012; Bulow et al., 2001), head and neck cancer (Lazarus et al., 2002), neurological degenerative diseases (Sprouls et al., 2013, and dysphagia from undefined causes (Lin et al., 2003). In healthy elderly, an effortful swallow resulted in an increase in hyo-laryngeal trajectory, and increased extent and duration of UES opening during the swallow (Hind et al., 2001; Jang et al., 2015). In individuals with head and neck cancer, effortful swallow resulted in an increase in the duration of base of tongue to posterior pharyngeal wall contact resulting in greater swallow pressure. Individuals with degenerative disease (e.g., OPMD) have been shown to have better organized and stronger swallow with the use of effortful swallow (Sprouls et al., 2013).
Extended use of the effortful swallow may have a restorative impact on swallow strength. The use of this technique as a rehabilitative approach is discussed in the next section.
Multiple Swallows
The use of multiple swallows per bolus may aid in bolus clearance. The technique may be spontaneously applied by patients with adequate sensory input regarding oral or pharyngeal retention. While there are no controlled studies evaluating the effectiveness of this technique to reduce pharyngeal residue, there is anecdotal evidence from videofluorographic evaluation that multiple swallows may reduce pyriform sinus retention (Palmer, Neel, Sprouls & Morrison, 2010).
Breath Holding
There are two maneuvers used in dysphagia treatment where breath holding is employed to close the airway —the supraglottic and super-supraglottic swallow (Box 4.23). The supraglottic swallow was so named because it was initially proposed as a treatment to improve airway protection in patients lacking in supraglottic structures (i.e., status post supraglottic laryngectomy). While this technique halts respiration, it does not consistently result in complete glottal closure. Thus, the super supraglottic procedure, which is a modification of the supraglottic swallow, adds a forceful breath-hold to increase the chance of achieving glottal closure. The goal of either technique is to improve airway safety before and during the swallow.
Box 4.23: Breath-holding maneuvers
| Supraglottic Swallow | Super Supraglottic Swallow |
|
1. Take a deep breath and hold it. a. Encourage individual to breathe in through 2. Take a bite of food / sip of liquid. 3. Swallow and continue to hold your breath 4. Cough immediately after the swallow. 5. Swallow again. 6. Breathe. |
1. Take a deep breath and then press hard (bear down) while holding your breath. a. You may provide the individual with the image you are lifting a heavy object and bearing down b. You may have individuals take a deep breath and then begin to vocalize before holding 2. Take a bite of food/a sip of liquid. 3. Swallow and continue to hold your breath until the swallow is completed. 4. Cough immediately after the swallow. 5. Swallow again. 6. Breathe. |
These maneuvers were designed for patients that have dysphagia characterized by late vocal fold closure or a delayed onset of the pharyngeal swallow. As these maneuvers provide improved airway protection, they are useful for any individuals with airway penetration or aspiration, regardless of presence or absence of supraglottic structures. These techniques may also be applied to individuals with premature spillage.
In the supraglottic swallow, the user is instructed to hold the breath before the swallow and maintain the breath hold throughout the swallow. In the super supraglottic swallow the addition of bearing down before the breath-hold recruits tighter vocal fold approximation, more closely approximated false vocal folds, and a forward tilt at the level of the arytenoids towards the base of the epiglottis (Logemann, Pauloski, Rademaker, & Colangelo, 1997). It also increases tongue-to-palate pressure (Fujiwara et al., 2014). Both breath-hold procedures are followed by a cough to encourage clearance of any airway residue.
To adequately perform this procedure an individual should be alert and be able to follow simple directions. During the training phase, saliva swallows should be trialed first. Ultimately the maneuver is applied to water swallows and eventually all swallows during mealtime. The instructions, while simple, require coordination and practice. Optimal training combines practice with biofeedback to supplement performance. Biofeedback choices may include a visual display of airflow, or for patients who can tolerate nasal endoscopy, the airway can be directly visualized during the swallow. During the training phase, asking the patient to push or pull against their chair may increase resistance during the chosen breath-hold maneuver.
Not all patients are appropriate for a breath-hold maneuver including individuals with limited cognitive ability, respiratory compromise, or history of hypertension (Rosenbek & Jones, 2008; Chaudhuri et al., 2002). Due to the complexity of the maneuver and the risk associated with poor performance, patients with reduced cognitive function are not appropriate for this maneuver. Patients with severe respiratory problems should be discouraged from using breath holding maneuvers, particularly when breath holding results in a drop in oxygen saturation or breathlessness. Individuals with coronary heart disease, uncontrolled hypertension, or history of stroke, should not use these maneuvers. The use of bearing down to generate a forceful breath hold is associated with an increased risk of triggering cardiac arrhythmias and sudden cardiac death (Chaudhuri et al., 2002).
Evidence to support the use of breath-hold maneuvers to reduce the immediate risk of penetration and aspiration is conflicting (Bulow et al., 2001; Logemann, et al., 1997; Logemann, Rademaker, Pauloski, & Kahrilas, 1994; Logemann et al., 1994). The ability for the techniques to improve swallow safety may be a function of patient selection. For example, in patients with head and neck cancer who undergo surgical resection, successful use of the technique may be related to the size of the resection (Zuydam et al., 2000). As with other maneuvers, the success should be assessed for a specific individual.
Long-term benefits of this procedure have not been evaluated in the literature. However, there is evidence that use of both supraglottic and super-supraglottic swallow increase tongue-to-palate pressure (Fujiu-Kurachi et al., 2014). Over time it is possible that this may increase tongue strength.
Sensory Enhancement
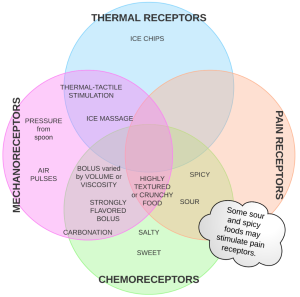
As sensory input is an essential aspect of triggering a successful swallow, it is no surprise that reduced oropharyngeal sensory function leads to dysphagia. Ideally, by providing sensory enhancements to patients with dysphagia who have reduced sensory function, swallow function should be positively impacted. That is, increasing or enhancing sensory input either through temperature, taste, texture, or pressure will modulate the response to a bolus (De Lama Lazzara, Lazarus, & Logemann, 1986) (Box 4.24). The oral cavity is rich with sensory receptors. The posterior oral cavity, particularly the faucial pillar region, contains receptors that feed into the nucleus tractus solitarius (NTS). It is postulated that increased sensory input will result in greater input to the swallow central pattern generator, which in turn increases the drive to the nucleus ambiguous. This heightens and facilitates faster triggering of the pharyngeal swallow (Power et al., 2006; De Lama Lazzara et al., 1986). Simply stated, oral sensory stimulation may lower the threshold required to trigger the swallow, thereby giving a more immediate reaction to the food stimulus. Sensory inputs can be incorporated into the bolus (e.g., a heavy bolus or pressure from the presenting spoon, cold or spicy foods, and carbonation) or delivered prior to the bolus (e.g., air pulses).
Box 4.24: Sensory enhancements
Clinical Note
Sources of a cold probe in order of cost
- Chilled 00 laryngeal mirror
- Chilled TTS specific metal probe
- Chilled long handled spoon with the end of the handle bent at a 90% angle
- Ice finger (cylindrical ice cube tray)
- Glove finger filled with water and frozen
Thermal Receptor Enhancements
Thermal (cold) stimulation has been used to increase sensory awareness in pediatrics and adults. Thermal stimulation is often combined with tactile stimulation as it is difficult to apply direct stimulation of cold without pressure. The two most commonly-used approaches are thermal-tactile stimulation (TTS) and ice massage.
Thermal-Tactile Stimulation
Of all the sensory enhancement techniques, TTS has received the most attention. TTS, sometimes referred to as thermal-tactile application, is a treatment that, as the name implies, combines pressure with thermal input to increase sensory awareness. This technique is used for individuals with reduced oral sensory function leading to a delayed pharyngeal swallow.
Thermal-tactile stimulation has been provided using a small (size 00) chilled laryngeal mirror to repeatedly stroke the anterior faucial pillars prior to food or liquid presentation. Other cold probes have been used including chilled spoon ends or ice sticks. When using this treatment procedure with pediatrics try an iced lemon glycerin swab or an iced NUK brush. While the stimulation site is typically described as the anterior faucial pillar, swallow timing also improves with contact to the uvula, base of tongue, or tonsils (Pereira, Motta, & Laélia, 2008).
TTS is typically provided by the clinician, patient or caregiver, before, and perhaps, even intermittently during, mealtimes. The cold probe is placed on the anterior faucial pillar on one side and stroked, top to bottom, multiple times. The probe is then removed and rechilled, if needed, before providing stimulus to the remaining anterior faucial pillar. Although there is no standardized procedure, a common protocol includes 3 to 5 strokes on each side to complete a set. A minimum of five sets can be performed easily in under 5 minutes. Between sets patient should be encouraged to swallow. This may be accomplished with the salivary buildup from the stimulus, or with small sips of water. After the 5 sets are completed, the patient should be given a bolus to swallow.
 Caution should be noted as the procedure, while rare, may trigger a syncope event, or a vasovagal or gag response. Therefore, this approach may not be appropriate for patients with a history of syncope or a strong gag. It is also difficult to complete this technique with patients who have excess movements, such as choreas, or restricted oral opening.
Caution should be noted as the procedure, while rare, may trigger a syncope event, or a vasovagal or gag response. Therefore, this approach may not be appropriate for patients with a history of syncope or a strong gag. It is also difficult to complete this technique with patients who have excess movements, such as choreas, or restricted oral opening.
The evidence to support the use of this technique as a compensatory approach to improving timing deficits is limited and inconclusive. Efficacy data has focused largely on the use of this technique with patients who have suffered a stroke, but there are also data to support the use of this technique in patients with neurodegenerative diseases (Regan, Walshe, & Tobin, 2010) and patients being treated for head and neck cancer (Lazarus, Clark, Arvedson, Schooling, Frymark, 2011). Efficacy of this procedure varies as a function of participant group. Not surprising, in healthy individuals who already have adequate response time, TTS does not significantly alter pharyngeal response time (Ali, Laundi, Wallace, deCarle, & Cook, 1996; Bove, Mansson, & Eliasson, 1998). However, a cold stimulus does increase cortical activity in healthy individuals (Magara, Watanabe, Tsujimura, Hamdy & Inoue, 2018). In patients with dysphagia, in general an immediate and transient effect is noted in pharyngeal response time including a more immediate trigger (i.e., reduced pharyngeal delay time) and shorter pharyngeal transit times (Rosenbek, Robbins, Fishback, & Levine, 1991; Rosenbeck, Roecker, Wood, & Robbins, 1996, Cola et al., 2012; De Lama Lazzara et al., 1986; Hwang, Choi, Ko, & Leem 2007; Regan et al., 2010). Further, there is increased cortical activation with TTS (Teismann et al., 2009). Of concern is that despite positive changes in timing, aspiration rates may not be reduced with the use of TTS (Selinger, Prescott, & McKiney, 1990).
Ice Massage
Ice massage is commonly used in sports injuries to reduce swelling. It has also been used to improve sensory awareness in oral structures by massaging the posterior oral cavity with an ice stick. Although very similar to thermal-tactile stimulation discussed above, it encourages cold stimulation to the anterior faucial pillars, as well as other posterior oropharyngeal structures including, for example, base of tongue and posterior pharyngeal wall. In patients with neurogenic dysphagia, response time was faster immediately after ice massage (Nakamura & Fujishima, 2013).
Taste Receptor Enhancements
Gustatory stimulation has been used to improve swallow function under the hypothesis that enhancing the flavor will more likely target and excite chemo-receptors in the oral cavity improving the alerting signal to the swallow pattern generator (Box 4.25). While multiple flavors have been explored, sour bolus and carbonation have received the most attention. Of interest here is that these stimuli may impact more than taste receptors. Sour may also stimulate pain receptors; carbonation also engages mechanoreceptors. Other tastes have been explored although at a much lower incidence in the literature. These include salty, sweet, and bitter (Ding, Logemann, Larson, & Rademaker, 2003; Yahagi, Okuda-Akabane, Fukami, Matsumoto, & Kitada, 2008; Leow, Huckabee, Sharma, & Tooley, 2006). In general, the addition of taste to a bolus increases sensory response.
Box 4.25: Impact of taste on swallowing

There is no one procedure employed for the use of taste as a sensory enhancement. Procedures vary from intermittent exposure to a flavor throughout mealtime to the use of impregnated boluses.
As with all compensatory strategies, the results are hypothesized to be immediate and transient. Age, gender, and other variables such as smoking status or presence of xerostomia, may play a role in the effectiveness of taste as a stimulation. Smaller bolus volumes may reduce the effect of taste as an effective stimulus (Miura, Morita, Koizumi, & Shingai, 2009; Pauloski et al., 2013). Bolus consistency may also impact the perception and reaction of a taste stimulant (Leow et al., 2006). Patients with central damage (e.g., status post stroke) may react differently to taste stimulus than patients with peripheral damage (e.g., status post oral surgery or treatment for head and neck cancer) (Pauloski et al., 2013).
Sour Bolus
The use of a sour bolus has been explored as a means to alter swallow timing. A sour stimulus engages taste receptors; nocioceptive receptors may also be stimulated with sour taste increasing the potential receptor field input to the central pattern generator. Like other sensory enhancements, the sour bolus is used to elicit a quicker and stronger trigger of the pharyngeal swallow. This response may be altered with age, which results in increases in the threshold required to respond to a sour bolus (Yamauchi, Endo, Saki, & Yoshimura, 2002).
Presentation of the sour bolus varies across studies, which may impact the effect it has on swallow timing. Studies that used single boluses tend to show shortened swallow times with quicker responses. The opposite is noted in studies that use continuous large drinking, such as when a sour bolus is added to a timed water swallow test. It is important to note that high concentrations of sour stimulus are considered by many to be unpalatable, and this may impact the speed at which the bolus is swallowed. This fact should be considered when designing a treatment protocol.
The presence of sour may impact both healthy individuals and individuals with dysphagia. However, evidence is inconsistent and depends on the patient population and bolus presentation format. In healthy individuals, using EMG, a sour bolus has been shown to increase muscle activation (Palmer, McCulloch, Jaffe, & Neel, 2005; Ding et al., 2003; Loew, Huckabee et al., 2007), and compress swallow timing (Palmer et al., 2005; Sciortino, Liss, Case, Gerritsen, & Katz, 2003; Ding et al., 2003). Yet, using scintigraphy, Alves and colleagues (2013) reported no change in timing. In individuals with dysphagia, the sour bolus increased swallow efficiency by reducing oral transit times and trigger delay (Logemann et al., 1995). Although use of a sour bolus has been shown to reduce the incidence of aspiration (Pelletier & Lawless, 2003). Careful consideration should be used before employing this approach in patients who have frank aspiration due to the impact of acidic contents on lung tissue. In summary, for patients with dysphagia bitter and sour boluses have been shown to reduce the duration of oral preparation (Neyraud, Peyron, Vieira, & Dransfield, 2005), bolus volume per swallow (Hamdy et al., 2003; Chee, Arshad, Singh, Mistry, & Hamdy, 2005), bolus volume swallowed per second (Chee et al., 2005) and pharyngeal delay of trigger response (Logemann et al., 1995).
There is no current literature to support the use of sour bolus as a rehabilitative approach. However, if consistent greater contraction is achieved with this approach, it is plausible that long term use will result in greater muscle function over time.
The outcome of this sensory enhancement may be improved when combined with other sensory enhancements, such as thermal stimulation. Iced tart lemonade or lemon Italian ice have been used with anecdotal success. While the approaches are logical, there are limited data to support or negate the effects of these combinations of sour and cold. However, in support of this notion, Sciortino and colleagues (2003) noted that in healthy individuals, the combined enhancements of sour, mechanical, and cold provided the greatest impact on swallow timing.
Carbonation
Carbonation has been proposed as an alternative to thickened liquids for individuals who aspirate on thin liquids. It is hypothesized that the use of carbonation increases sensory input to chemoreceptors sensitive to carbonic acid, and mechanoreceptors. The sensory impact of carbonation may reduce with bolus volume as bubbles may dissipate quickly with smaller bolus volumes (Miura et al., 2009).
Patients of all ages with reduced sensory input and slowed response time (i.e., delayed trigger) may benefit from carbonated beverages as a stimulus. Patients on thickened liquids who may not be receiving adequate hydration and have complained that the thickened liquids aren’t palatable, may be more accepting of carbonation and gain a similar benefit to thickened liquids. As with all compensatory approaches, carbonation is best employed when benefits have been confirmed during evaluation.
Carbonation may be a poor choice for individuals with reflux as it encourages belching. Also, because carbonation is not a stable stimulus, it may be less effective in individuals with slowed motor patterns. Individuals who have protracted mealtimes may not get consistent support from carbonation as a sensory enhancement. Over the course of a mealtime, carbonation weakens as the bubble disperse, thereby reducing the mechanical sensory stimulus and relying solely on the sensory response to the carbonic acid.
In individuals with healthy swallow function, carbonation produces transient brainstem excitation (Magara, Michou, Raginis-Zborowska, Inoue, & Hamdy, 2016) and greater tongue-to-palate pressure (Krival & Bates, 2012). Yet, carbonation does not consistently impact muscle activation or swallow timing in healthy individuals (Morishita, Mori, Yamagami, & Mizutani, 2014; Miura et al., 2009; Ding et al., 2003). In adults with neurogenic dysphagia, there is promise for the use of carbonation as a treatment to improve swallow response time and reduce the incidence and depth of penetration and aspiration (Bulow, Olsson, & Ekberg, 2003; Sdravou, Walshe, & Dagdilelis, 2012). However, across studies carbonation does not always translate to reductions in the number of aspiration events in pediatric patients (Newman et al., 2001; Lundine, Bates, & Yin, 2015).
Clinical Note
Carbonation excites both chemoreceptors via carbonic acid and mechanical receptors via the carbonation bubbles.
Mechanoreceptor Enhancements
Intraoral tactile stimulation is used: (1) to prevent oral hypersensitivity in infants and children who are unable to have oral intake, (2) to improve recovery of swallow function in patients who require long-0term intubation, and (3) during mealtimes to increase sensory drive to the swallow pattern generator.
Clinical Note
Changes in bolus viscosity are not only useful for sensory enhancement. If a bolus is more viscous it may have greater cohesion. For example, consider the bolus cohesion of rice versus pudding. If a bolus stays together, it will put a reduced motor load on the oral cavity to contain and channel it down the correct pathway.
Clinical Note
While the use of a viscous cohesive bolus may reduce the risk of penetration, when penetration does occur, it may be harder to eject.
The use of tactile stimulation as an alerting signal to provide sensory enhancement for the pharyngeal swallow can be achieved through food presentation or food selection. During food presentation, the delivering utensil can be pressed on the tongue before dispensing the food. When food is used as the tactile stimulus, increasing levels of crunchy and irregular foods (e.g., pretzels or varying shapes and thickness, cheese puffs of varying hardness) that are safely tolerated can be used to provide oral stimulus.
Efficacy data is limited and typically uses transition to, or return to complete oral feeding as an outcome measure. In infants who have had little or no experience with oral intake, intraoral stimulation serves as an aid to transition from the feeding tube (Fucile et al., 2002; Coker-Bolt et al., 2013). For patients who are intubated for greater than 48 hours, oral stimulation may improve recovery of swallow function post intubation (Hwang et al., 2007).
Clinical Note
In healthy individuals, combined sensory enhancements have proven to be more effective than single sensory enhancements with respect to timing and kinematics (Sciortino et al., 2003; Hamdy et al., 2003). However, in contract, cortical excitation as a result of stimulation is largest with single-sensory stimuli.
Air Pulse
Although not well researched or even used clinically, air pulses to the posterior oral cavity have been investigated and shown to improve swallow response in stroke patients. Limited data shows that for some patient’s an intraoral air pulse increases the rate of swallowing (Theurer et al., 2013). It should be noted that the air pulses may alter the moisture level of the mucosa. It is unclear if this treatment should be avoided in patients with known xerostomia, such as patients with peripheral damage to salivary glands (e.g., patients who received radiation treatment for head and neck cancer).
Bolus Volume & Viscosity
While bolus variables such as thickness and volume may be used to alter the bolus flow, they can also be employed to provide sensory enhancement by increasing the sensory load on oral afferents. The number of receptors that interact with the bolus and the amount of deformation or rate of flow may alter the level of excitability.
Bolus volume can be increased or decreased as a treatment approach to improve swallow safety. Smaller bolus volumes may be more manageable in the oral cavity, thereby reducing risk of penetration or aspiration. Larger bolus volumes may increase sensory awareness and improve trigger response time. Swallow accommodations noted when a larger bolus volume is used include increased UES open duration (Nascimento, et al., 2015), improved pharyngeal response time (reduced delay) (Bisch et al., 1994; Logemann et al., 1995), increased duration of swallow apnea (Butler, Postma, & Fischer, 2004), and increased pharyngeal pressures (Butler et al., 2009). Care should be taken when choosing to employ a larger bolus volume, as this may increase penetration and reduce swallow efficiency and safety (Clave et al., 2006; Butler et al., 2011).
Clinical Note
Compensatory strategies may be combined in a variety of ways, such as combining postures with diet changes (Kagel & Leopold, 1992; Fukuoka et al., 2013; Wheeler-Hegland, Rosenbeck, & Sapienza, 2008).
Clinical Note
The term oral aversion may be used to describe not only oral defensiveness, but any avoidance or fear of eating, drinking, or accepting sensation in or around the mouth. It may occur not only from the lack of oral feeding experience, but may also be related to discomfort from gastrointestinal disorders or respiratory issues, sensory regulation disorders, or a history of choking episodes.
The effectiveness of bolus characteristics as a sensory enhancement may be a function of patient diagnostic group (Clave et al., 2006; Lan et al., 2017; Inamoto et al., 2013; Hiss & Huckabee, 2005; Dantas et al., 1990; Rogus-Pulia, Pierce, Mittal, Zecker, Logemann, 2015; Perlman, Schultz, & VanDaele, 1993; Butler et al., 2009; Nagy et al., 2015; Kendall, Leonard, & McKenzie, 2001). Although there is limited timing data, changes in viscosity have been shown to reduce the extent of penetration and aspiration in individuals with dysphagia due to stroke, brain injury, or head and neck cancer (Newman, Vilardell, Clavé, & Speyer, 2016; Clave et al., 2006;). Increased viscosity has also been linked to greater amplitude of EMG in the oropharynx, and increased pressure in the pharyngeal and esophageal regions (Reimers-Neils, Logemann, & Larson, 1994; Watts & Kelly, 2015; Omari, Dejaeger, Tack, VanBeckevoort, & Rommel, 2013).
Oral Stimulation
The use of tactile stimulation to prevent the development of hypersensitivity for non-oral patients can be achieved with a consistent oral stimulation program. When patients are unable to receive oral intake, there is reduced swallow experience. In these cases, intraoral massage or tactile stimulation may serve to prevent hyper-sensitivity of the system. To stimulate the oral-pharyngeal structures, massage the cheeks, lips, gums, and tongue. (See Fucile, Gisel, & Lau, 2002 for a detailed description of an oral stimulation program for non-oral infants. This is also covered in greater detail in the Infant Feeding section of this chapter.) The massage of these structures can be done using firm touch, muscular elongation (for the tongue), special oral brushes (e.g., NUK brush or toothbrushes with varying levels of hardness), or vibration. When long term tube feeding results in oral hypersensitivity or oral sensory defensiveness, tactile stimulation may be used to reduce the hypersensitivity.
Other Alerting Enhancements
Sucking or chewing may serve as an alerting signal that a swallow is soon needed. That is, an over-exaggerated suck or chewing pattern during the oral phase can be used to initiate the pharyngeal swallow. This has been used in infants, as well as individuals with dysphagia status post head injury.
Mealtime / Environmental Alterations
Both the mealtime and treatment environments may be manipulated to improve swallow physiology. This may be useful for individuals with limited attention or those who are easily distracted, such as infants, or individuals with developmental delay or brain injury. Environmental alterations may include feeding in a quiet room with low lights and reduced distractions. In cases where a feeder is used, the alterations may include the presentation or approach used while feeding. For example, tapping the lips with the spoon before entering the oral cavity, placing the spoon on the side with better sensory/ motor function, or pressing the spoon firmly on the mid-tongue before emptying its contents. Identifying and employing an environment conducive to maximal swallow function may improve swallow safety and efficiency.

