Signs and Symptoms of Oropharyngeal Dysphagia
Clinical Signs & Symptoms
Clinical signs and symptoms of dysphagia are varied, ranging from the obvious, such as choking, to the subtle, such as facial grimacing during mealtimes. The presence of a sign or symptom does not guarantee a swallow disorder or the presence of aspiration. However, specific signs and symptoms associated with dysphagia may be identified during effective screening for dysphagia. Box 2.1 provides examples of some commonly reported symptoms and their clinical signs.
Box 2.1: Signs and symptoms that may be observed during the dysphagia exam

Clinical Note
Symptoms are reported by the patient.
Signs are observed or measured during the clinical or instrumental exam.
Sensitivity is the percent of patients with a disease who test positive for that disease.
Specificity is the percent of patients without a disease who test negative for that disease.
To assess the predictability of a single sign or symptom to be a sensitive and specific indicator of the presence of dysphagia or aspiration, findings on clinical examinations are compared to instrumental findings. Box 2.2 summarizes sensitivity and specificity for various clinical signs and symptoms. Interpretation of this data should be considered with respect to: (1) the level of sensitivity and specificity, individually and combined, (2) the consistency of these variables across multiple investigations, and (3) the patient population used in the investigation. Box 2.2 is not exhaustive. Vomiting, throat clearing, presence of stridor, and eye tearing have also been reported to be linked to dysphagia (Benfer et al., 2015; DeMatteo, Matovich, & Hjartarson, 2005; Weir, McMahon, Barry, Masters, & Chang, 2009).
Box 2.2: Summary of sensitivity and specificity of various clinical signs and symptoms

Sensitivity or specificity greater than .75 is bolded.
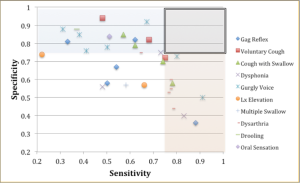
Although clinical signs and symptoms can help us identify a risk of dysphagia, that information alone is insufficient to draw direct physiologic implications. Box 2.3 displays sensitivity versus specificity for individual signs and symptoms across the studies presented in Box 2.2. In Box 2.3 high specificity is indicated by the blue shading; high sensitivity is indicated by the pink shading. Overall, individual signs and symptoms lack consistent sensitivity and specificity across investigations. In fact, across the literature, sensitivity (the ability to identify people with a disease) and specificity (the ability to identify people without a disease) does not yield an obvious single clinical variable that consistently has both a high sensitivity and high specificity. Using a cut off of .75 in Box 2.3, the box that highlights the overlap of these two shaded segments indicates that while some variables come close, no single variable has both high sensitivity and specificity as a predictor for risk of dysphagia. Assessing the presence of multiple signs and symptoms, serves as a better predictor of risk than a single symptom alone.
Box 2.3: Summary of sensitivity and specificity across various signs and symptoms of swallowing
Clinical Note
Signs and symptoms associated with dysphagia include:protracted mandible, impaired oral sensation, oral incontinence or drooling, nasal regurgitation, weak voice, weak neck muscles, residue or stasis, oral or pharyngeal pain or globus sensation.
Presence or Absence of a Gag Reflex
The gag reflex is a brainstem reflex that may be elicited by a foreign or unwanted stimulus entering the oropharynx (Box 2.4). The result is a strong contraction with elevation of the velum, pharynx, and larynx. In patients with dysphagia, an abnormal gag reflex has a sensitivity ranging from 33-62% and a specificity ranging from 81-82%. In short, an abnormal gag reflex may be an indicator of dysphagia (Daniels et al., 1998). Yet, a healthy gag reflex is not required to produce a safe swallow. In fact, the gag reflex is absent in 4-13% of non-dysphagic individuals (Leder, 1996; Ramsey, Smithard, & Kalra, 2005). That is, the individual may not produce a gag response (CN X), despite feeling the stimulus (CN IX). In summary, an intact gag reflex does not imply a healthy swallow, nor does an abnormal gag reflex guarantee a swallow deficit. Although the presence of an abnormal gag reflex does indicate a higher likelihood of dysphagia. Consideration should be given to those individuals who not only have an absent gag response, but also deny feeling the stimulus.
Box 2.4 Video: Visualization of stimulated gag reflex
Click to play the video. Note that the velum elevates and the tongue protrudes.
Clinical Note
Recall that the gag reflex receives sensory input from CN IX and generates a motor response via CN V and X. Therefore, the lack of an observed gag reflex does not definitively define sensory status.
Clinical Note
In adults, the gag reflex should be tested on both the right and left side. Regardless, if intact, the soft palate should elevate symmetrically.
“Want more?”
Currently, there are no data comparing the strength of a healthy reflexive cough to a voluntary cough in healthy individuals (Magni, Chellini, Lavorini, Fontana, & Widdecombe, 2011).
Coughing
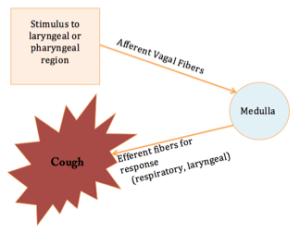
A cough is a brainstem reflex whose purpose is to protect the airway (Box 2.5). Specifically, if material penetrates the laryngeal vestibule, a cough will be reflexively triggered to expel material out of the airway entrance. A weak cough may render the individual unable to expel foreign material from the larynx. Coughing during mealtimes may be linked to penetration of material into the laryngeal vestibule (Daniels et al., 1998; Logemann, Veis, & Colangelo, 1999). In individuals with swallow disorders, the spontaneous cough may be weak or absent. If the penetrated material is not properly ejected and goes below the true vocal folds, it is considered aspiration. In the case of an absent cough, the individual is at risk for silent aspiration — entering below the vocal folds without a clinically observable physiologic response. In individuals with dysphagia, spontaneous coughing during mealtimes has a sensitivity for identifying dysphagia ranging from 57-78%. For individuals who are status post stroke, a weak voluntary cough has a specificity for dysphagia ranging from of 58-85%. In other words, individuals with a strong and functional voluntary cough are less likely to have dysphagia.
Box 2.5: Schematic of a cough reflex
Clinical Note
Spontaneous coughing does not always mean that an individual has dysphagia. Cough not associated with mealtimes may be related to medication (e.g., ACE inhibitors such as Lisinopril) or pulmonary disease (e.g., asthma). This symptom is also noted in frail and weak elderly individuals, but is not necessarily related to dysphagia
While a spontaneous cough during oral intake implies oropharyngeal swallow deficits, it is possible to have swallow deficits and not stimulate a spontaneous cough. When this happens, the clinician may ask the individual to produce a voluntary cough and use the strength of that cough to judge its potential effectiveness. A voluntary cough may provide insight into the strength of the structures used in the production of a reflexive cough. A weak voluntary cough has a sensitivity for the presence of dysphagia of 48-68% and a specificity of 82-94%.
General Dysphonia or Wet Vocal Quality
Individuals with dysphagia may have a wet or gurgly voice quality associated with swallowing, especially during and immediately after mealtimes. This is true in both adults and children (Weir et al., 2009) and is generally linked to moderate to severe penetration or aspiration (Weir et al., 2009; Irwin, 2006). (See Box 2.6 for a sample of a gurgly vocal quality.) The physiologic assumption is that if material collects in a supraglottic or pharyngeal space, and remains there after the swallow, then voice quality will be altered during phonation. Material in the supraglottic region implies that either the individual is lacking in sensory function (risk of silent aspiration), or motorically weak, such that a cough is inadequate to expel the laryngeal contents. Regardless, the end result is the risk of aspiration after the swallow. Overall, the sensitivity of a gurgly voice quality during or after oral intake ranges from 38-91%; specificity ranges from 40-92%. Dysphonia (with or without a gurgly vocal quality) is associated with dysphagia. The presence of dysphonia has a sensitivity of 48% and a specificity of 56% as a risk marker for dysphagia.
Box 2.6 External Link
Drooling
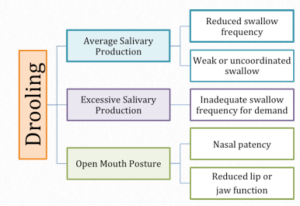
Drooling, or anterior loss of oral contents, can occur during mealtimes, as well as throughout the day. Although drooling is considered normal in infants and toddlers due to either immature muscle tone or increased salivation associated with tooth eruption, the presence of drooling may also indicate a deficit. Box 2.7 depicts common causes of drooling. Drooling may occur when there is a mismatch between the amount of saliva produced and the frequency or functionality of saliva swallows. Other causes of drooling include inadequate sensory or motor function of the lips, or poor nasal patency resulting in an open oral posture for breathing. Logemann and colleagues (1999) reported the sensitivity and specificity of drooling as an indicator of dysphagia to be 37% and 88%, respectively. When drooling is observed, an attempt should be made to (a) assess the cause, and (b) quantify the amount. The Drooling Severity Scale depicted in Box 2.8 (Rashnoo & Daniel, 2015) is one example of a way to quantify the extent of drooling.
Box 2.7: Common causes of drooling
Box 2.8: Drooling Severity & Frequency Scale

(Rashnoo & Daniel, 2015)
Dysarthria
Dysarthria and dysphagia have been shown to co-occur with neuromuscular diseases (Knuijt et al., 2014). Oral motor strength required for intelligible speech, at least with respect to the tongue, is much less than that required for a safe and efficient swallow (Neel & Palmer, 2012; Neel, Palmer, Sprouls, & Morrison, 2015). Thus, the presence of dysarthria may imply inadequate strength to produce a functional swallow. (Box 2.6 links to a YouTube video of a patient with dysarthria. Note the gurgly vocal quality.) Recall that the swallow is a pressure-driven event. If oropharyngeal muscle strength is reduced, then pressure generation may be impaired. The end result is an insufficient pressure to effectively and safely propel the bolus through the oral and pharyngeal cavities. Residue may occur, risking aspiration after the swallow. The sensitivity and specificity of dysarthria as an indicator of dysphagia ranges from 64-78% and 44-75%, respectively.
Complaint of Pain with Chewing or Swallowing (Odynophagia)
Odynophagia, pain while swallowing, may be reported by individuals with dysphagia. However, odynophagia is more often associated with an infection in the mouth or throat, such as candidiasis or herpes. Non-infectious causes, such as ulcers, tumors, or gastroesophageal reflux disease (GERD), should also be considered. If it is a young individual or someone with reduced cognitive function, consider the possibility of a foreign object in the throat, particularly if the caregiver reports a sudden onset (and a missing item). Odynophagia may be accompanied by difficulty breathing (dyspnea) and/or chest pain.
When pain with swallowing is reported, localization of pain should be assessed and may be as simple as asking the patient to point to the site that hurts. There are some caveats to consider when asking an individual to localize pain. First, localization skills may be limited in individuals with cognitive impairment or in the pediatric population. Second, pain or discomfort from the esophagus may be referred to the hypopharynx. For example, esophageal reflux is commonly associated with discomfort in the hypopharynx. In cases of referred pain, individuals may report that something is “stuck” in the hypopharynx, yet nothing is visualized on instrumental exam. This globus sensation, as it is sometimes called, is linked to reflux and may co-occur with chest pain associated with meal times (or just after mealtimes).
Clinical Note
While localization of pain may indicate the region in need of careful visual inspection, not all anatomic regions associated with swallow can be clinically visualized. Instrumental exam or referral to an ENT or GI physician may be required.
Clinical Note
Do not confuse odynophagia (pain with swallowing) with globus sensation (a sensation of tightness in the throat).
Multiple Swallows per Bolus
Individuals with oropharyngeal dysphagia may swallow multiple times for a single bolus. Although a large bolus may necessitate a piecemeal approach, it is possible that an inefficient and weak swallow may result in reduced bolus clearance with a single swallow per bolus. The remaining bolus residue may require multiple swallow attempts to complete the transport of the entire bolus through the oral and pharyngeal cavities, even with small bolus amounts. Multiple swallows are common in patients with neuromuscular disease when pharyngeal function is weak (Palmer, 2010; Palmer, Neel, Sprouls, & Morrison, 2010). Multiple swallows as an indicator of dysphagia has a sensitivity and specificity of 58 and 57%, respectively.
Changes in Oral Intake, Weight Loss, or Inadequate Weight Gain
Oral intake is the food and liquid taken in by mouth and not lost anteriorly. Insufficient oral intake increases the risk of: (1) inadequate weight gain or growth in infants and children, or (2) weight loss and malnutrition in adults. While adequate growth and weight gain for infants and toddlers are well defined in commonly-used growth charts, clinically relevant weight loss in adults is not as easily determined. As a general rule, weight loss is considered clinically relevant if there is a loss of more than 5% of the body weight across a 6 month period (Gaddey & Holder, 2014). Reductions in oral intake or progressive unintentional weight loss may indicate the presence of dysphagia. Clinically relevant weight loss, or reductions in oral intake that threaten weight maintenance, should result in a referral to a nutritionist or other appropriate medical professional. If oral intake is deemed unsafe, or if oral intake does not provide adequate nutrition, then alternate means of nutrition (such as tube feedings) may be used as a supplement to feedings or in place of oral feeding.
 Failure to Thrive
Failure to Thrive
Failure to thrive (FTT) is a term that can refer to either infants or toddlers who fail to achieve anticipated growth, or adults who experience a state of decline that leads to weight loss and malnutrition. Common causes of FTT are indicated in Box 2.9. FTT in infants may be the result of inadequate oral intake or poor absorption of oral intake. In the latter scenario, an illness or medical condition may alter the gut’s ability to extract nutrients, thus reducing growth. When FTT is a result of poor oral intake, this may indicate poor feeding ability or food intolerance. FTT is common in preterm infants; approximately 40% of preterm infants exhibit abnormal or immature feeding patterns (Hawdon, Beauregard, Slattery, & Kennedy, 2000; Mercado-Deane et al., 2001). This may be related to: (1) reduced in-utero sucking practice, and (2) reduced oral-motor development and muscle tone. Further, preterm babies may have medical complications that interfere with adequate feeding or limit the ability to coordinate the suck-swallow-breathe pattern. FTT may have long-term consequences on growth and development. However, early treatment and support may minimize developmental and cognitive differences between kids with FTT and their healthy peers.
Box 2.9: Common causes of failure to thrive
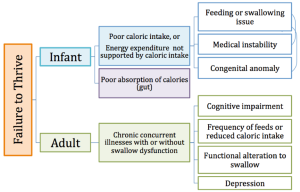
FTT in adults describes a state of decline whose cause is related to multiple variables and may include concurrent chronic illnesses in combination with functional alterations to the swallow. Whether or not dysphagia is associated with adult FTT, weight loss, reduced appetite, reduced activity level, and poor nutrition are noted in these individuals. Some individuals may be identified as cachexic, which implies severe muscles wasting (much greater than age-related sarcopenia) due to chronic illness.
Congenital Anomalies
Congenital anomalies, especially those associated with the oropharyngeal cavity or airway, may contribute to feeding and swallowing deficits. Box 2.10 lists the consequences of various head and neck anomalies on swallow function. An infant with a cleft lip and palate may have difficulty generating the needed pressure to adequately draw liquid from a nipple without excess work, negating the nutritional benefits of feeding. An infant with a laryngeal cleft or tracheo-esophageal fistula may have an increased risk of aspiration. These typically require surgical intervention before oral intake and feeding development can be encouraged (Ha, Wertz, Driver, Zopf, & Ha, 2018).
Box 2.10: Incidence of congenital anomalies of the head and neck with their impact on feeding

For adults, changes in oral intake that results in unintentional weight loss may be related to dysphagia. Consideration should be given to changes in medical or mental status, changes in oral or pharyngeal function, or cognitive decline. Not all changes in oral intake are related to swallowing disorders. Identifying the cause of reduced oral intake will aid in selecting the proper referrals, as well as inform dysphagia treatment, if needed.
Clinical Note
Regardless of the etiology of poor growth, when a child is already small, dropping a growth curve (e.g., from 10% to 5%) is detrimental to their health and growth at a time when important growth is taking place.
Supplemental and Alternate Means of Nutrition
The presence of alternate means of nutrition may indicate that an individual has an underlying swallow or feeding disorder. Alternate means of nutrition may be used for full nutritional intake or for supplemental feeding, and may be employed for individuals who have clinically relevant weight loss, FTT, or inadequate cognition, anatomy or physiology to safely take food and liquid orally. Alternate means of nutrition can be divided into enteral and parenteral nutrition (Box 2.11). When alternate nutrition is required, enteral nutrition is the usual, and by far, the preferred mode of nutrition. Enteral nutrition allows nutrients to be absorbed by way of the gastrointestinal tract. This approach may be used when the oropharyngeal and/or esophageal aspects of the alimentary canal need to be bypassed for safety or to ensure adequate weight gain.
Box 2.11: Alternate means of nutrition
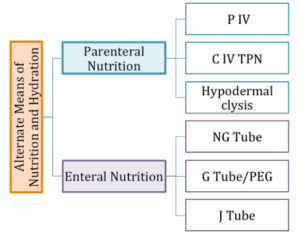
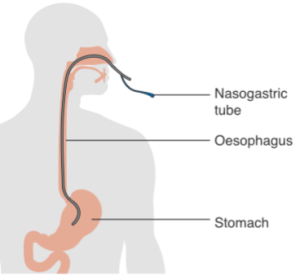
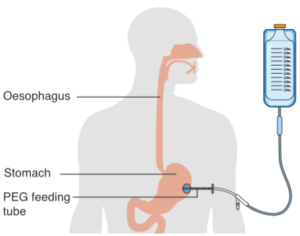
When enteral nutrition is employed, nutritional material is introduced to the stomach or gastrointestinal tract through the use of a tube (Box 2.12). Various routes include placing a feeding tube through a nostril (i.e., a naso-gastric tube or NG tube) or oral cavity (i.e., oro-gastric tube), or piercing through the abdominal skin directly into the stomach (i.e., a gastrostomy tube or G-tube, percutaneous endoscopic gastrostomy or PEG) or intestines (i.e., jejunostomy or J-tube).
Box 2.12: Alternate means of enteral nutrition
| NG-Tube | PEG | J-Tube |
 |
 |
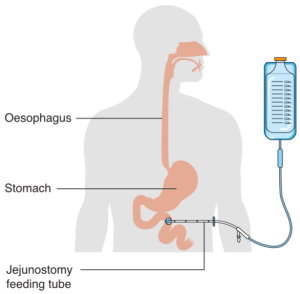 |
By Cancer Research UK –(Original email from CRUK) [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia
Parenteral nutrition bypasses the alimentary canal and directly introduces needed nutrients into the bloodstream. When total parenteral nutrition (TPN) is used, hypertonic solutions are delivered by a central intravenous (CIV) line. A peripheral IV (PIV) line can be used to deliver partial parenteral nutrition (PPN). Parenteral nutrition is typically reserved for patients with significant gut problems (such as an obstructed bowel or short bowel syndrome), or when there is a short-term immediate need (such as dehydration due to illness). To protect the gut, parenteral nutrition is converted to enteral routes as soon as medically feasible.
Clinical Note
PEG tubes may be used prophylactically in patients where you anticipate swallow deficits, such as patients who will undergo radiation or chemo therapy (Shaw et al., 2015).
“Want more?”
Check out this ASHA FAQ on Alternate Means of Nutrition and Hydration.
Although the speech-language pathologist (SLP) does not aid in the selection of alternate nutrition, the SLP may provide the evidence for the need of such an approach by diagnosing a level of dysphagia that denotes reduced safety with oral intake. Note that alternate routes for nutrition do not eliminate the risk of aspiration; aspiration may still occur on oral saliva or refluxed material. In fact, the presence of the feeding tube may increase the risk of oropharyngeal deficits. Further, extended use of feeding tubes may hinder swallow ability (Langmore, Krisciunas, Miloro, Evans, & Cheng, 2012).
Clinical Note
Enteral nutrition may be associated with increased risk of GERD, as well as aspiration (Ferrer, Bauer, Torres, Hernández, & Piera, 1999; Schallom, Orr, Metheny, & Pierce, 2013).
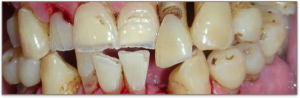
Poor Oral Care
Good oral care has been linked to better general health and a reduced risk of illness. Conversely, poor oral care is linked to increased health risks. Oral health can be achieved with routine oral care including brushing and flossing. For individuals with dysphagia or sensory deficits, the cleansing routine should also include checking oral pockets in order to remove any bolus residue and debris. If left unattended, bolus residue after the swallow may putrefy and build bacteria resulting in notable halitosis (bad breath). Visual inspection of the oral cavity can provide evidence of the adequacy of oral care. If poor oral care is present and aspiration of saliva occurs, the individual is at higher risk for pneumonia. In the event of micro-aspiration (aspiration of small amounts of material), the dense and concentrated bacterial load imposed by poor oral care increases the risk of aspiration pneumonia (Langmore et al., 1998). Additionally, poor oral health is associated with an increased risk of cardiovascular disease, heart attacks, and strokes (Dai et al., 2015).
Clinical Note
In at-risk populations for poor oral care, such as toddlers, or motorically or cognitively impaired adults, oral care should be delivered by trained personnel who understand the importance of good oral care.
The variables that lead to poor oral care may be age-dependent. At an early age, oral care may be limited with respect to frequency (number of times per day that the teeth are brushed and the mouth is rinsed) and number of teeth contacted (the thoroughness of oral cleaning events). In the geriatric population, oral care may decline due to limited motor function and strength, reduced vision, and perhaps even reduced cognitive function. When an individual can not adequately perform oral care, the task maybe completed by a caregiver. Risks associated with poor oral care may be related to the skill of the person administering the oral care. In some cases, the connection between poor oral care and aspiration pneumonia is related to the dependence on a caregiver for oral care (Langmore et al., 1998). However, evidence suggests that when oral care is performed by a trained health care professional, the oral contents have a reduced bacterial load (Yoshino, Ebihara, Ebihara, Fuji, & Sasaki, 2001; Matsuo et al., 2016). This is likely due to differences in the quality of the oral care. Regardless, when thorough oral care is performed on a routine basis, it reduces the risk of aspiration pneumonia (Müller, 2015).
Oral care may not be the only culprit when dealing with oral bacterial load. Dental decay, gum disease, and reduced saliva may encourage bacterial buildup in the oral cavity. In summary, good oral care not only provides protection against the consequences of micro-aspiration, but it may also stimulate the physiologic system resulting in improved swallow physiology (Yoshino et al., 2001; Watando et al., 2004; Poisson, Laffond, Campos, Dupuis, & Bourdel-Marchasson, 2016).
 Frailty
Frailty
Frailty, commonly noted in the very elderly population, refers to increased vulnerability or a decline in functional reserve across multiple physical systems (Xue, 2011; Lee, Auyeung, Leung, Kwok, & Woo, 2014; Morley et al., 2013). Frailty may be an outcome from dysphagia (van der Maarel-Wierink, Vanobbergen, Bronkhorst, Schols, & de Baat, 2011). Specifically, frailty has been associated with impaired tongue strength and propulsion, as well as poor airway protection. Frail individuals are more likely to have trouble with swallowing when new foods or textures are introduced. Further, frail individuals are more likely to have a negative response to even small amounts of aspiration.
Clinical Note
The definition of frailty is usually associated with the following criteria: weight loss, self-reported exhaustion, a low level of physical activity, slow walking speed, and motor weakness (typically assessed via hand grip).
Instrumental Signs
While some clinical signs and symptoms can be easily linked to swallow physiology during the clinical exam, clinical signs that imply pharyngeal deficits are best assessed through the use of instrumental exams that visualize the oral and pharyngeal system during the dynamic act of swallowing.
Oropharyngeal Bolus Transfer
Typically, in a healthy individual, a cohesive bolus is transported from the horizontal to the vertical pathway in a single event. When this does not occur, the dysphagia clinician will assess the contribution of piecemeal deglutition, premature spillage of bolus from the oral cavity into the pharynx, or delayed pharyngeal trigger of the bolus (Box 2.13). Differentiation of piecemeal deglutition, premature spillage, and delayed or absent trigger can be performed by observing the bolus flow characteristics with respect to the amount of bolus and the level of coordination in the transfer from the horizontal to the vertical bolus trajectory, as well as the onset of hyo-laryngeal elevation associated with a swallow.
Box 2.13: Comparison of oral-pharyngal bolus transfer mechanisms

Piecemeal Deglutition
At times, particularly if the bolus is large, the bolus will be parsed into segments, where each individual segment is transported to the pharynx and swallowed, while the remaining bolus is purposefully and safely retained in the oral cavity. This process of piecemeal deglutition is controlled by the individual and occurs without incident. In healthy individuals, piecemeal deglutition may occur in boluses greater than 20ml. When this pattern is noted in smaller bolus sizes, the individual may have dysphagia (Ertekin, Aydoğdu, & Yüceyar, 1996).
Clinical Note
Premature spillage may be within normal limits in spontaneous swallows in elderly individuals. When premature spillage is observed consider the following swallow deficits: poor oral preparation or bolus control, poor oral transport, or delayed pharyngeal trigger.
Premature Spillage
Premature spillage is the unintentional entrance of a portion of the oral bolus into the pharyngal cavity during the oral preparation or transport (Box 2.14). This is typically the result of poor bolus control in the oral cavity, combined with poor lingual-velar valving (base of tongue and velar contact during the oral phase of the swallow). Because the stray piece of bolus falls into the pharynx before the pharyngeal phase has been readied, the swallow trigger may not occur in a timely fashion (or at all), placing the individual at risk for penetration into the airway before the initiation of the pharyngeal swallow.
Box 2.14 Video: VFES example of premature spillage and delayed trigger
Delayed or Absent Swallow Trigger
At times the bolus is intentionally transported to the pharynx, either as a single cohesive bolus or in pieces, yet the pharyngeal swallow is not triggered in a timely fashion (Box 2.14 and 2.15). This is referred to as a delayed trigger. Although a delayed or absent trigger may indicate dysphagia, a delayed trigger has been correlated with healthy aging. An impaired swallow trigger may be related to: (1) reduced sensory systems with inadequate information transported to the swallow CPG, (2) inadequate motor systems to carry out commands from the swallow central pattern generator, or (3) neurological involvement altering the capabilities of the central pattern generator. Because a timely swallow trigger correlates with the initiation of hyo-laryngeal elevation, and therefore airway protection, when the trigger is delayed or absent, there is an increased risk of penetration and/or aspiration of the bolus before the swallow.
Box 2.15 Video: Absent swallow trigger
Oral & Pharyngeal Residue
Oropharyngeal residue is a common occurrence in patients with dysphagia. Residue may be localized to one area or multiple discrete areas, or distributed throughout the oropharyngeal system. The location(s) of bolus residue may implicate the swallow deficit(s). Box 2.16 lists common residue sites with linkage to potential swallow deficits. In the oral cavity, residue is typically linked to poor bolus control, reduced lingual propulsion, or poor oral pressure generation. Localized oral residue can occur in the anterior or lateral sulci in the oral cavity, on the floor of mouth (under tongue), or coating the palate.
Box 2.16: Location of oropharyngeal residue and probably deficit
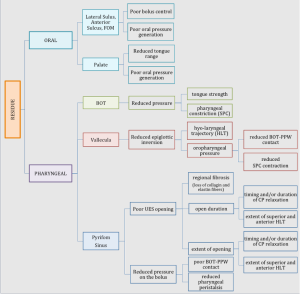
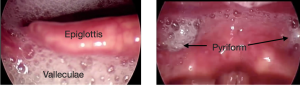
In the pharyngeal cavity, residue may be isolated to a region or distributed throughout. When evaluating the cause of the pharyngeal residue, the contribution of reduced hyo-laryngeal elevation and pressure generation should be considered. Coating of structures or pooling of the bolus can occur in the vallecula space, pyriform sinus, base of tongue, or posterior pharyngeal wall. Judgements of pharyngeal residue are impacted by the instrumentation used. Residue noted during FEES is judged to be greater than residue noted during VFES (Kelly, Leslie, Beale, Payten, & Drinnan, 2006). Box 2.17 and 2.18 reveal distributed pharyngeal residue.
Box 2.17: Endoscopic images of secretions in the pharynx
Box 2.18: Patient with distributed pharyngeal residue as observed on VFES.
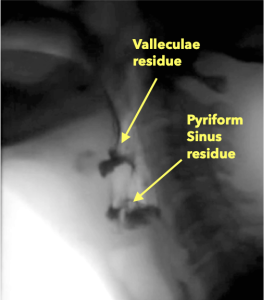
Isolated vallecula residue may suggest that epiglottic down-folding was incomplete or inadequate. Recall that the down-folding of the epiglottis (a cartilage) is achieved through activation of attached muscles combined with passive forces from pressure and mechanical alterations related to the superior-anterior hyo-laryngeal excursion. The trajectory of epiglottic down-folding can be sub-divided into 2 components—(1) from upright to horizontal and (2) inversion. The movement from upright to horizontal is accomplished by a combination of passive forces introduced by laryngeal elevation with limited assistance from contraction of the small aryepiglottic muscle fibers. Complete inversion is highly dependent on the pharyngeal driving pressures as the bolus traverses the pharynx. Reductions in laryngeal trajectory or pharyngeal pressure may result in inadequate epiglottic inversion limiting valleculae clearance.
Isolated residue in the pyriform sinus is more likely related to alterations in UES opening, either with respect to extent of opening or duration of opening. Recall that adequate UES opening is a function of input from pharyngeal pressure, relaxation of CP muscle, and passive opening from the trajectory of the hyo-laryngeal complex, particularly the anterior aspect of the trajectory. UES opening may be impacted by age due to a reduction in the number of elastin and collagen fibers which can alter the passive abilities of the UES in response to the hyo-laryngeal excursion. Pyriform sinus residue may implicate any or all of the mechanisms of UES opening.
Trace residue may adhere to and outline pharyngeal structures such as base of tongue or pharyngeal wall. Recall that adequate pharyngeal clearance is related to both the pharyngeal pressure generated from base of the tongue to pharyngeal wall contact and pharyngeal stripping from sequential constrictor activity. When coating exists along the pharyngeal wall, the pharyngeal pressure is suspected to be insufficient. At times, residue may be unilateral–located on one side or the other. In this case, unilateral deficits should be considered.
Velar Deficits
The velum serves as a valve that aids functional swallowing in two ways: (1) during the horizontal bolus path, the velum may contact with the base of tongue, thus preventing premature spillage, and (2) during the vertical bolus path, the velum actively closes off the nasal cavity, thus preventing nasal penetration.
When functioning as a lingual-velar valve (i.e., engaged with the base of tongue), the velum may help prevent leakage of oral contents into the pharynx during the development of the oral bolus and movement of the bolus from the anterior to the posterior oral cavity. Observation of premature spillage of oral contents into the pharynx before completion of bolus development may implicate inadequate oral function or dysfunction (sensory, motor, and/or coordination) of the lingual-velar valve.
As the bolus transfers from the horizontal to the vertical direction, the velum makes contact with the pharyngeal wall (velar-pharyngeal valve) to close off the naso-pharynx and prevent penetration of material into the nasal cavity. Additionally, the closing of this valve helps maintain adequate pharyngeal pressure to encourage downward propulsion. When nasal penetration is noted (as seen in Box 2.19), the strength, timing, and adequacy of the velar-pharyngeal valve should be assessed.
Box 2.19 Video: Nasal penetration as observed on VFES
Reduced Airway Protection
Poor airway protection can result in penetration or aspiration of material. (See examples of penetration and aspiration from VFES in Box 2.20.) During the swallow, airway protection occurs as a result of several actions. First, as a result of supraglottic muscular activation of the hyo-laryngeal complex, the airway is lifted in a superior-anterior position. The result is that the larynx is tucked under the jaw and out of the way of the bolus flow pathway. This trajectory can be manually palpated or visualized on VFES or FEES. Reduced hyo-laryngeal trajectory can be discussed with respect to inadequate superior and/or anterior aspects of the trajectory.
Box 2.20 Video: Airway invasion observed during VFES
Second, internal laryngeal closure, which includes vocal fold contact, prevents penetration from being aspirated into the airway. Laryngeal closure is further supported by aryepiglottic tightening and epiglottic down-folding which collectively serve to reduce the size of and cover the the laryngeal aditus.
Airway protection may be compromised before or after the swallow or at times other than mealtime. Poor oral control can lead to material falling into the airway before the swallow has been triggered (premature spillage), rendering the airway unready and unprotected. When pharyngeal residue remains in the oro-pharynx after the swallow, it may enter into the airway once the protective actions have ceased. Further, when oral care is lacking, even when an individual is not eating or drinking, remaining material or excessive secretions may be aspirated. Box 2.21 shows an endoscopic visualization of an impaired larynx where copious secretions enter below the vocal folds without obvious reaction from the individual.
Box 2.21 Video: Aspiration of secretions observed endoscopically
Poor UES Opening
The UES (a.k.a. the pharyngo-esophageal segment) is composed of elastin fibers and muscle fibers. During the swallow, this region opens to allow passage of the bolus from the pharynx to the esophagus. Optimal UES opening is achieved through a combination of passive and active forces. Due to the attachment of the UES to the posterior cricoid, movement of the larynx passively manipulates the UES opening. That is, upon contraction of the supraglottic musculature, the hyo-laryngeal complex is pulled in a superior-anterior direction. The attachment of the UES to the posterior larynx pulls the anterior fibers, thus stretching open the UES region.
The muscular (or active) component of UES opening is dictated by the cricopharyngeus muscle. This muscle is tonically active (closed) during most actions to provide redundant protection of the airway by blocking esophageal contents from entering into the pharynx. The cricopharyngeus muscle activity relaxes during the swallow allowing for easy opening of the UES region.
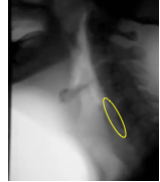
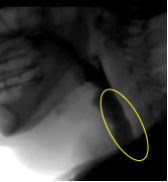
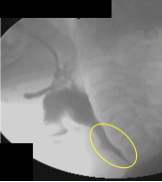
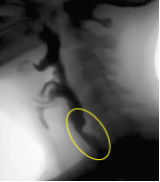
When the active and passive forces are well timed and supported by the driving pressure of the bolus, adequate opening is achieved. When inadequate opening is observed, it may be a result of an individual mechanism or a combination of impairments across the various opening mechanisms — active or passive forces, or bolus driving pressure. Box 2.22 displays images of the UES region before and during swallowing.
Box 2.22: UES opening under various swallow conditions and diseases
|
UES region BEFORE Swallow |
UES during 5 ml thin (s/p CVA) |
UES duing 5ml thin |
UES during 5ml nectar (note CP Bar) |
 |
 |
 |
 |


